8 th Grade Science Atomic Structure and Periodic





- Slides: 35

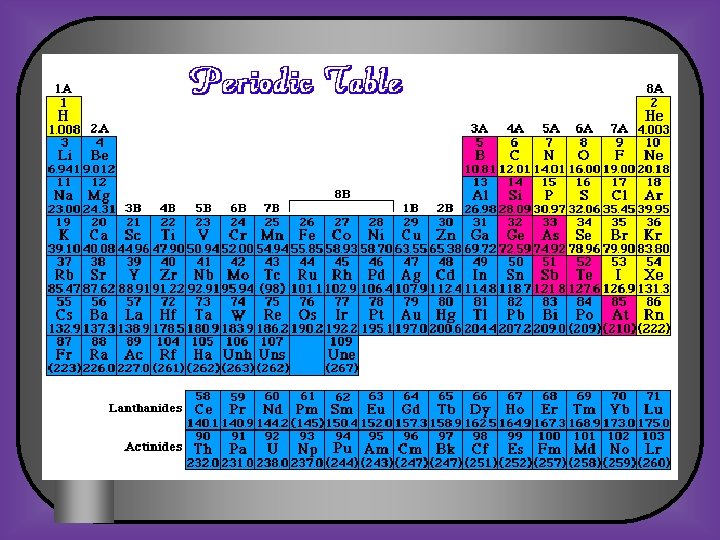
8 th Grade Science Atomic Structure and Periodic Table Review


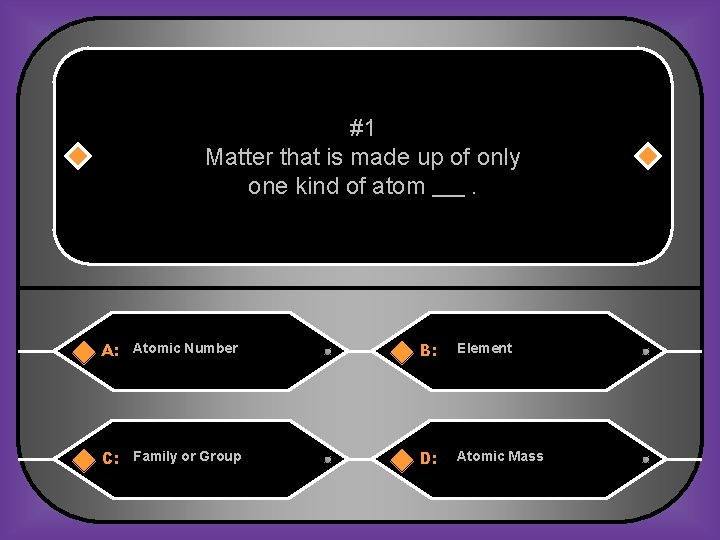
#1 Matter that is made up of only one kind of atom. A: Atomic Number B: Element C: Family or Group D: Atomic Mass

B. Element

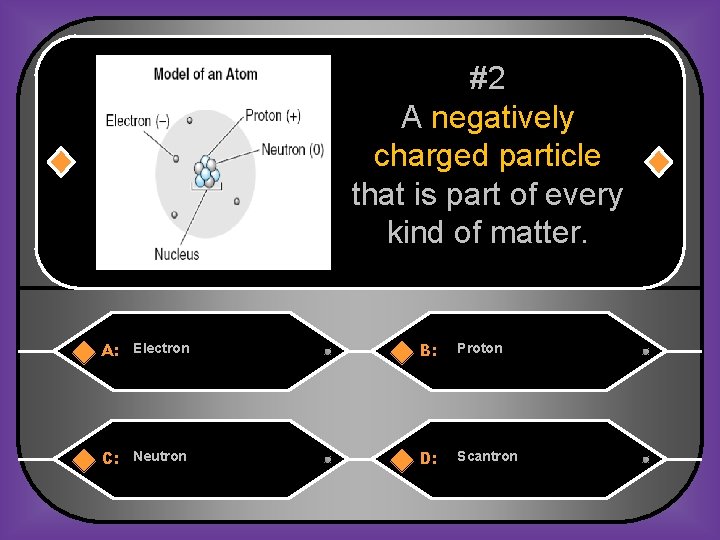
#2 A negatively charged particle that is part of every kind of matter. A: Electron B: Proton C: Neutron D: Scantron

A. Electron

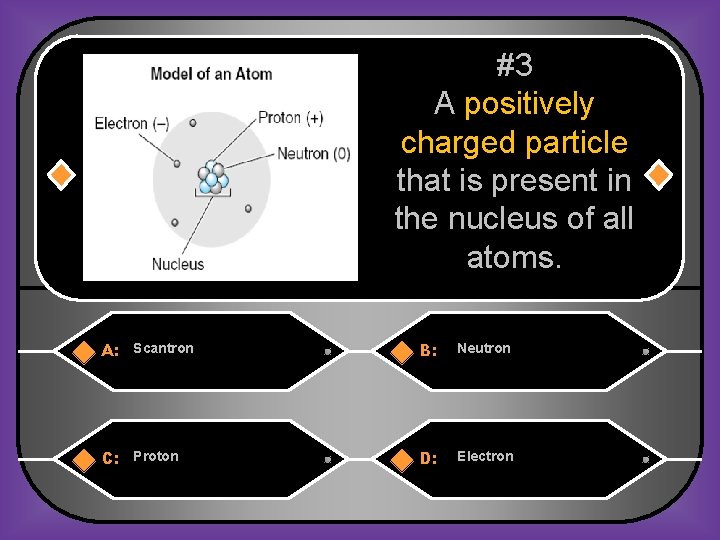
#3 A positively charged particle that is present in the nucleus of all atoms. A: Scantron B: Neutron C: Proton D: Electron

C. Protron

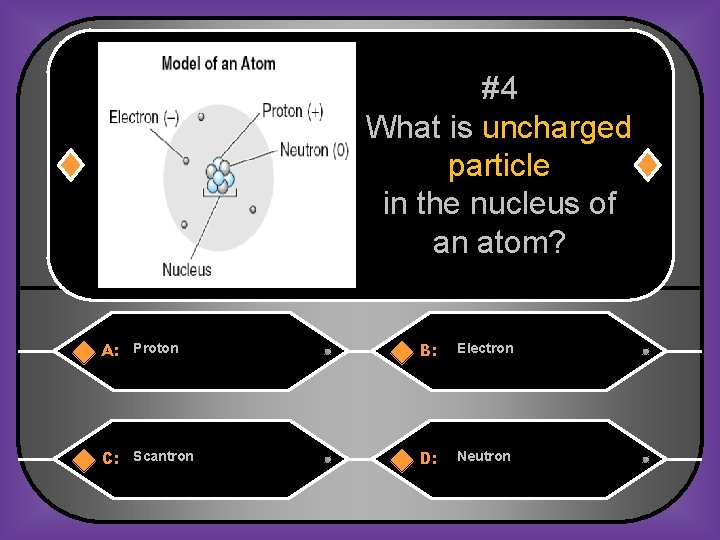
#4 What is uncharged particle in the nucleus of an atom? A: Proton B: Electron C: Scantron D: Neutron

D. Neutron

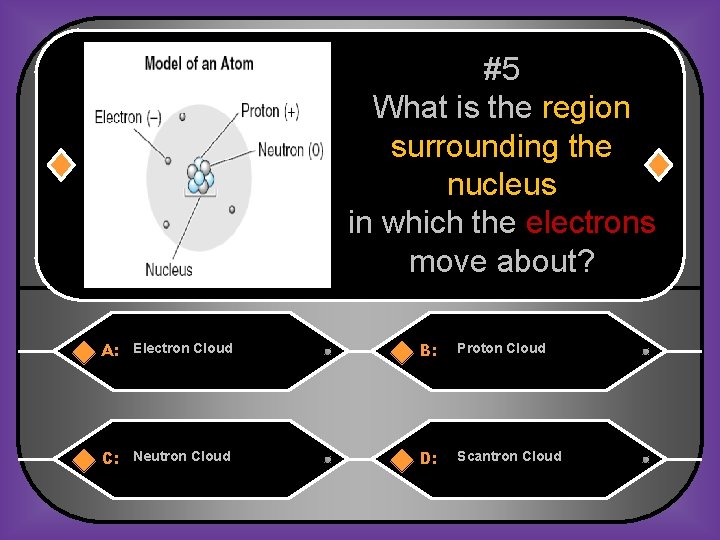
#5 What is the region surrounding the nucleus in which the electrons move about? A: Electron Cloud B: Proton Cloud C: Neutron Cloud D: Scantron Cloud

A. Electron Cloud

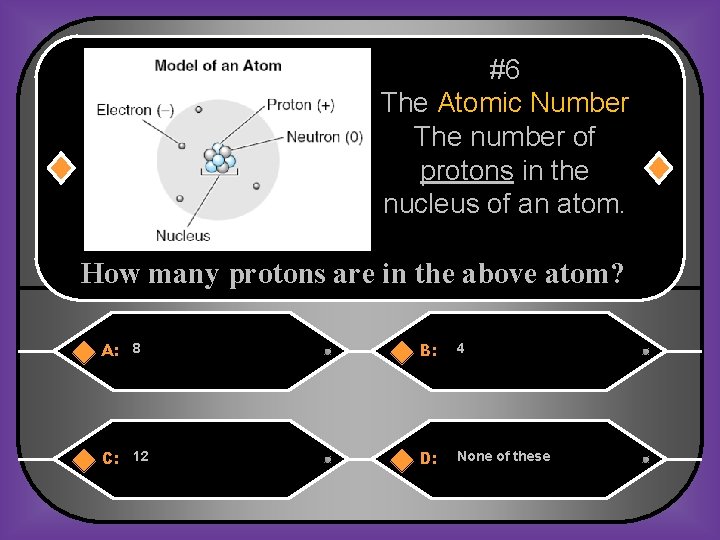
#6 The Atomic Number The number of protons in the nucleus of an atom. How many protons are in the above atom? A: 8 B: 4 C: 12 D: None of these

A. Atomic Number

#7 What is the number of neutrons plus the protons in the nucleus of an atom? Neutrons + Protons = ____ A: Atomic Number B: Atomic Matter C: Atomic Element D: Atomic Mass

D. Atomic Mass

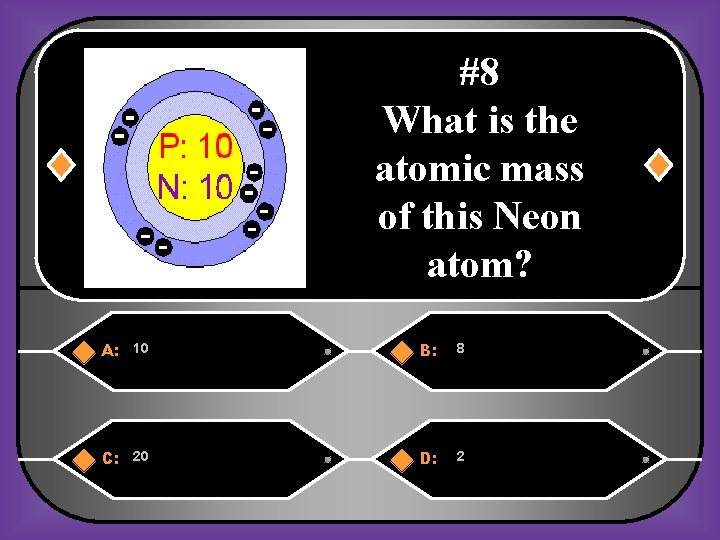
#8 What is the atomic mass of this Neon atom? A: 10 B: 8 C: 20 D: 2

C. 20

#9 What is an element that is malleable, ductile, has luster, and is a good conductor of heat and electricity. A: Gases B: Nonmetal C: Transition Metals D: Metal

D. Metal

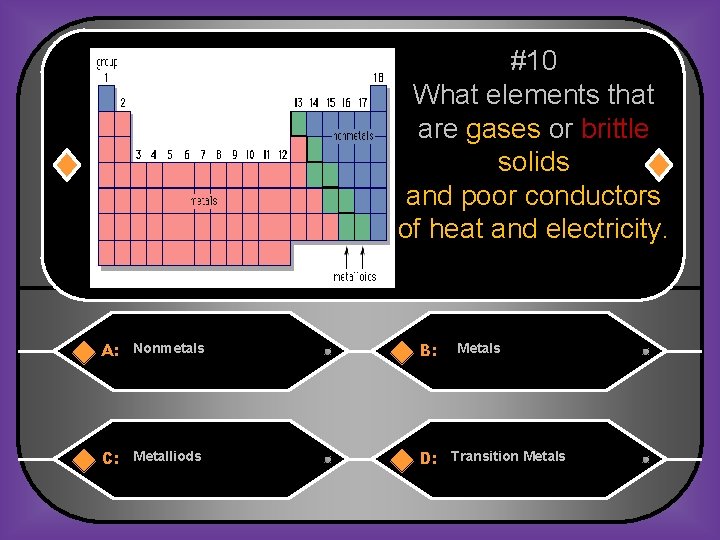
#10 What elements that are gases or brittle solids and poor conductors of heat and electricity. A: Nonmetals B: C: Metalliods D: Transition Metals

A. Nonmetals

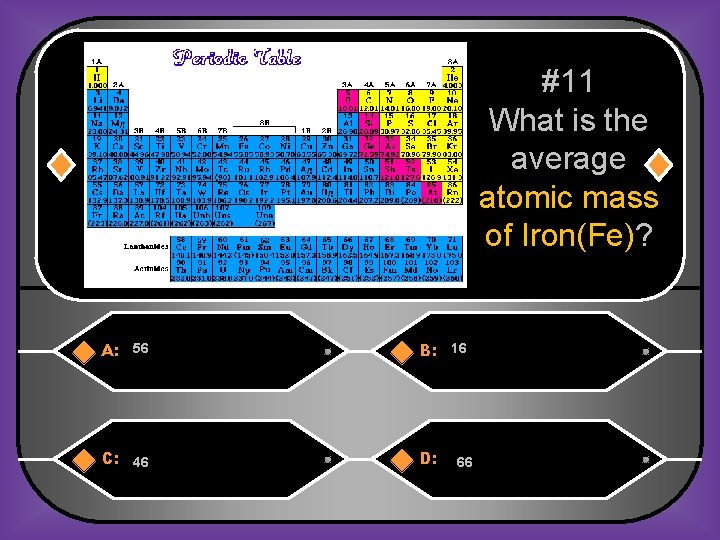
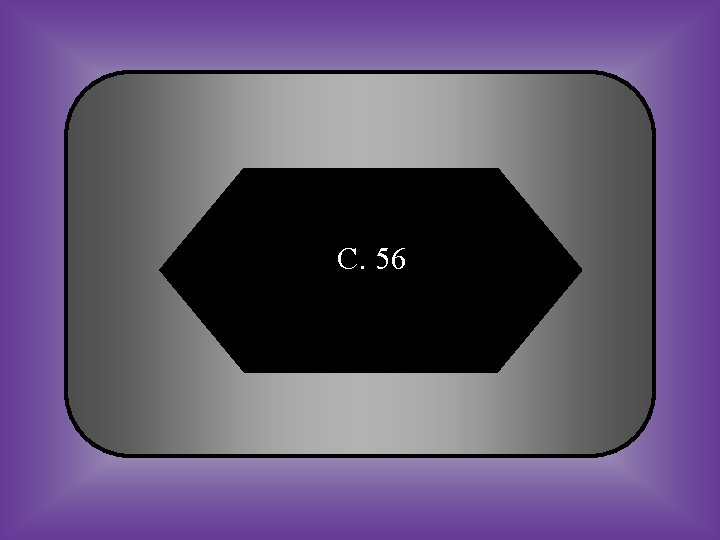
#11 What is the average atomic mass of Iron(Fe)? A: 56 B: 16 C: 46 D: 66

C. 56

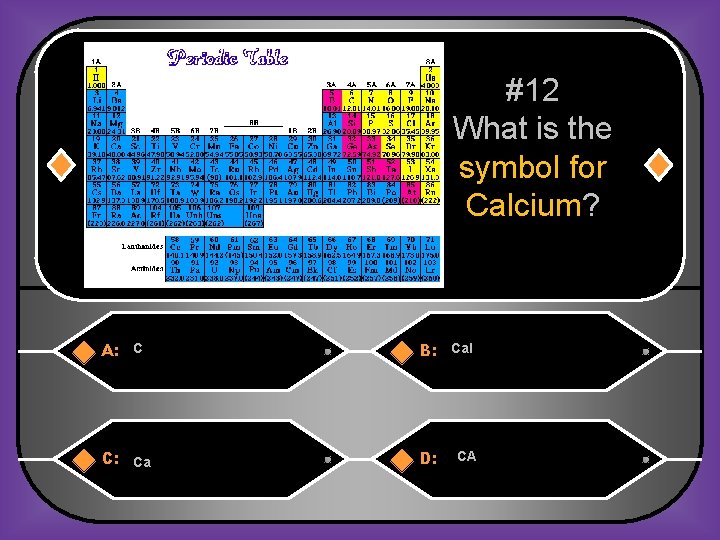
#12 What is the symbol for Calcium? A: C B: Cal C: Ca D: CA

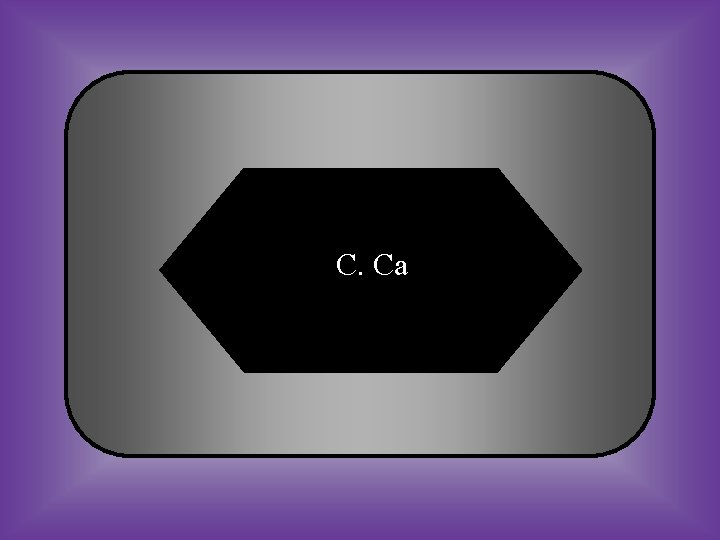
C. Ca

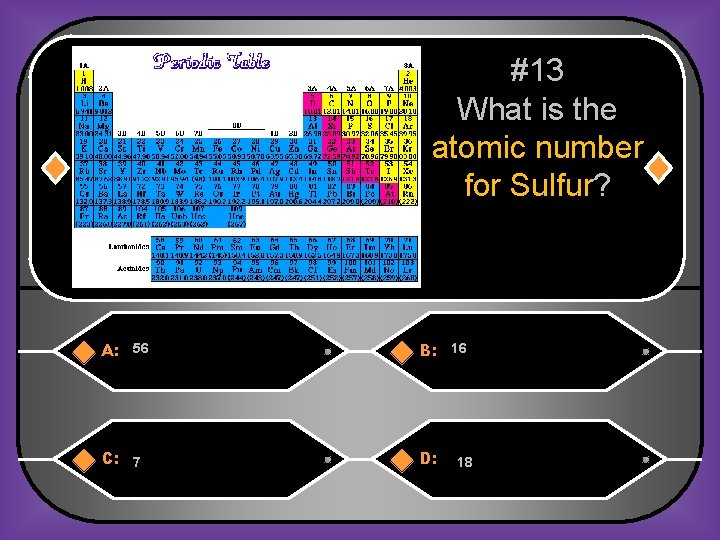
#13 What is the atomic number for Sulfur? A: 56 B: 16 C: 7 D: 18

B. 16

#14 What are the properties of nonmetals? A: C: Luster, brittle, low melting point, do not conduct heat or electricity. Dull, brittle, low melting point, conductor of heat or electricity. B: Dull, brittle, high melting point, do not conduct heat or electricity. Dull, brittle, low melting point, do not conduct heat or electricity.

D. Dull, brittle, low melting point, do not conduct heat or electricity.

#15 Elements in the Periodic Table arranged in order of increasing _____. A: Atomic Mass B: C: Atomic Number D: Family or Group Ductile

C. Atomic Number

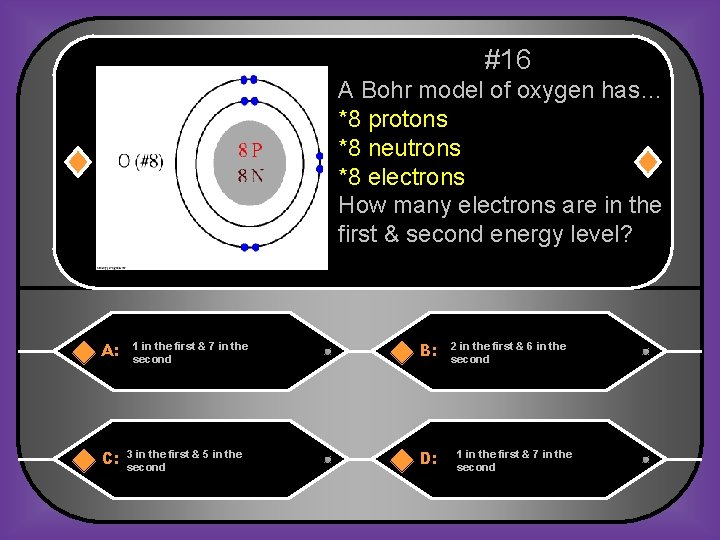
#16 A Bohr model of oxygen has… *8 protons *8 neutrons *8 electrons How many electrons are in the first & second energy level? A: C: 1 in the first & 7 in the second 3 in the first & 5 in the second B: D: 2 in the first & 6 in the second 1 in the first & 7 in the second

B. 2 in the first & 6 in the second

Great Job!!!! Thank you for playing!