8 8 Electric properties of colloids 1 Electrokinetic

![[(Ag. I)m Colloidal core · n I– · Surface charge (n-x)K+ ]x Compact layer [(Ag. I)m Colloidal core · n I– · Surface charge (n-x)K+ ]x Compact layer](https://slidetodoc.com/presentation_image_h/e04665931a48b28a2da3c935b3e608ad/image-7.jpg)
- Slides: 14

§ 8. 8 Electric properties of colloids

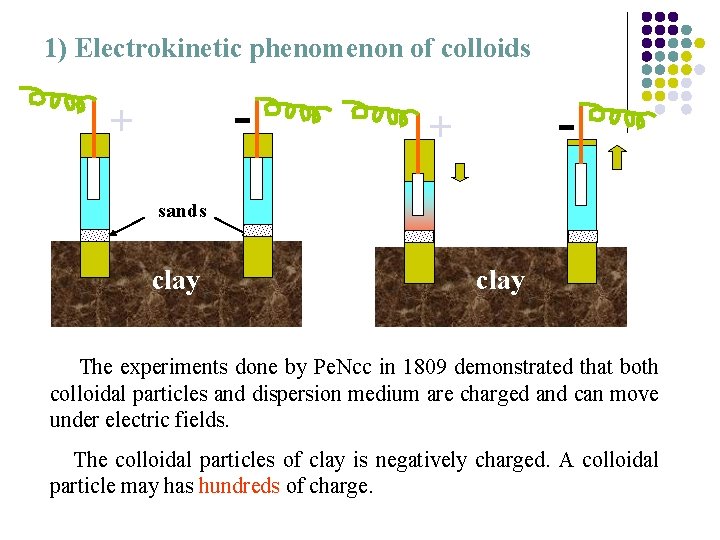
1) Electrokinetic phenomenon of colloids - + sands clay The experiments done by Pe. Ncc in 1809 demonstrated that both colloidal particles and dispersion medium are charged and can move under electric fields. The colloidal particles of clay is negatively charged. A colloidal particle may has hundreds of charge.

Electrokinetic phenomena: 1) Electrophoresis: 2) the motion of colloidal particles under the action of an electric field. 2) Electro-osmosis: the motion of dispersion medium under electric field

Positively charged sols: metallic oxide sol, metallic hydroxide sol and some dyes. Negatively charged sols: metal, metallic sulphide, sulfur, clay, paper, silicic acid. Some sol, such as Ag. I sol, can be either positively charged or negatively charged. Lyophilic sols (protein solution): can be positively, negatively charged or neutral depending on the p. H and the colloids.

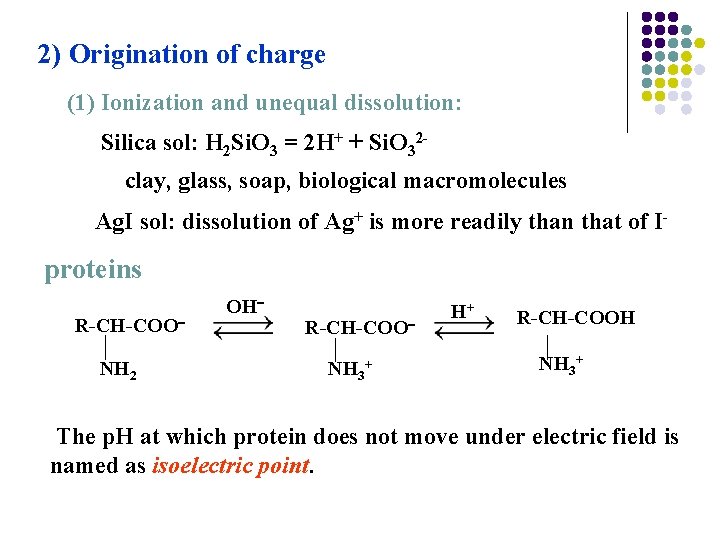
2) Origination of charge (1) Ionization and unequal dissolution: Silica sol: H 2 Si. O 3 = 2 H+ + Si. O 32 clay, glass, soap, biological macromolecules Ag. I sol: dissolution of Ag+ is more readily than that of I- proteins R-CH-COO NH 2 OH R-CH-COO NH 3+ H+ R-CH-COOH NH 3+ The p. H at which protein does not move under electric field is named as isoelectric point.

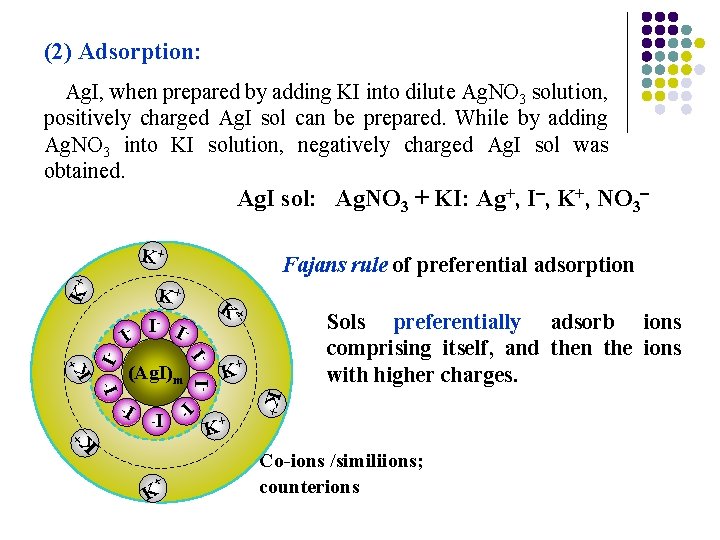
(2) Adsorption: Ag. I, when prepared by adding KI into dilute Ag. NO 3 solution, positively charged Ag. I sol can be prepared. While by adding Ag. NO 3 into KI solution, negatively charged Ag. I sol was obtained. Ag. I sol: Ag. NO 3 + KI: Ag+, I , K+, NO 3 K+ K+ Fajans rule of preferential adsorption K+ K+ I- I I Sols preferentially adsorb ions comprising itself, and then the ions with higher charges. + I- I- I - + (Ag. I)m K + I- I + K K K+ K I- I - - + K Co-ions /similiions; counterions
![Ag Im Colloidal core n I Surface charge nxK x Compact layer [(Ag. I)m Colloidal core · n I– · Surface charge (n-x)K+ ]x Compact layer](https://slidetodoc.com/presentation_image_h/e04665931a48b28a2da3c935b3e608ad/image-7.jpg)
[(Ag. I)m Colloidal core · n I– · Surface charge (n-x)K+ ]x Compact layer x K+ Diffusion layer Colloidal particle Colloid (3) Substitution of crystal lattice: Caolin: {[m(Al 3. 34 Mg 0. 66)(Si 8 O 20)(OH)4]0. 66 m-(0. 66 -x)Na+}x- x. Na+ (4) Dielectric difference Water droplet in petroleum is negatively charged.

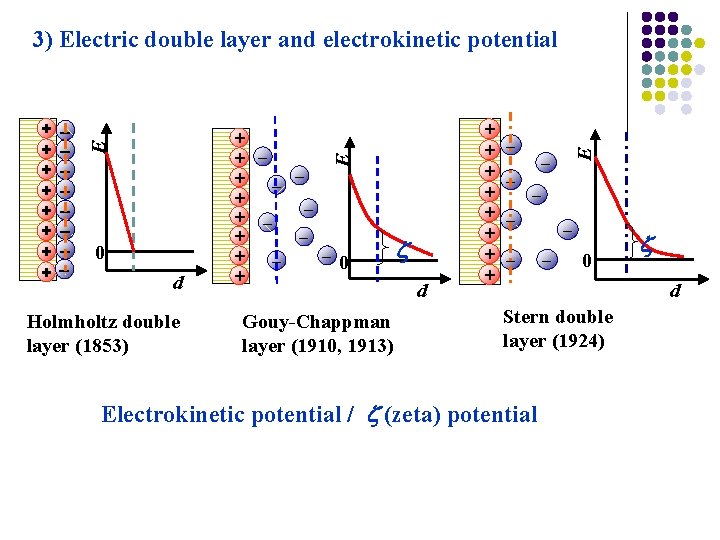
+ + + + + 0 d Holmholtz double layer (1853) Gouy-Chappman layer (1910, 1913) + + + + Plane+of + shear+ 0 + E E + + + + E 3) Electric double layer and electrokinetic potential d Stern double layer (1924) Electrokinetic potential / (zeta) potential d

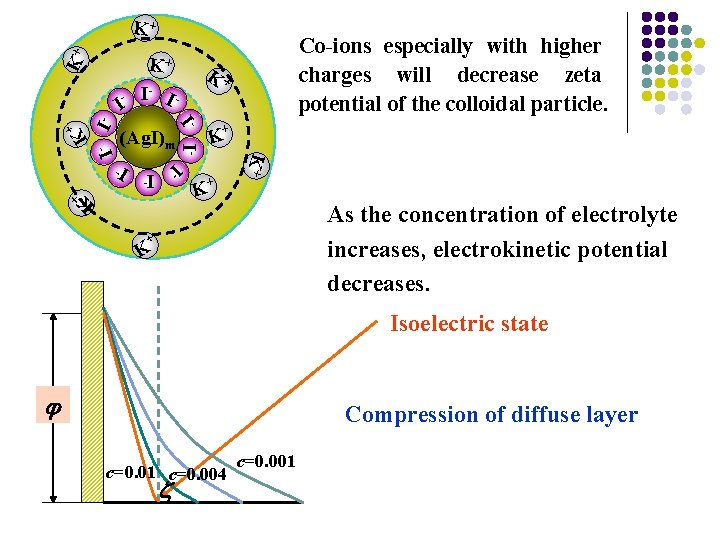
K+ K+ K+ I- + (Ag. I)m K + K+ I- I- I - K I- I - I- I- Co-ions especially with higher charges will decrease zeta potential of the colloidal particle. I- I K + K As the concentration of electrolyte increases, electrokinetic potential decreases. + K Isoelectric state Compression of diffuse layer c=0. 01 c=0. 004 c=0. 001

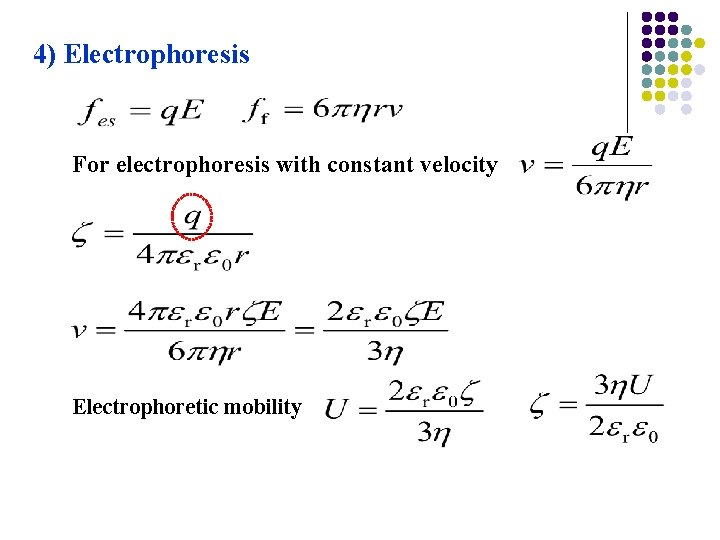
4) Electrophoresis For electrophoresis with constant velocity Electrophoretic mobility

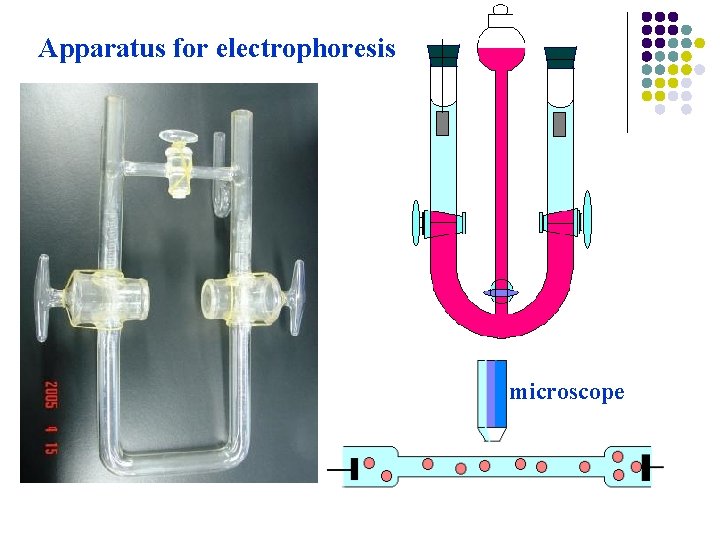
Apparatus for electrophoresis microscope

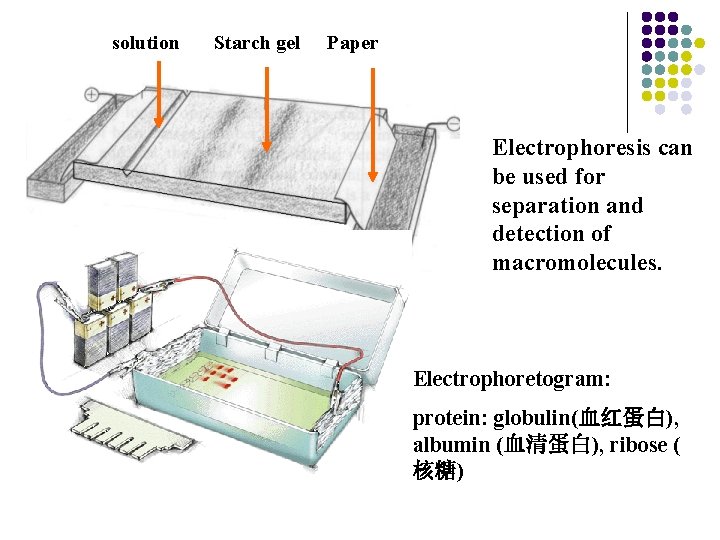
solution Starch gel Paper Electrophoresis can be used for separation and detection of macromolecules. Electrophoretogram: protein: globulin(血红蛋白), albumin (血清蛋白), ribose ( 核糖)

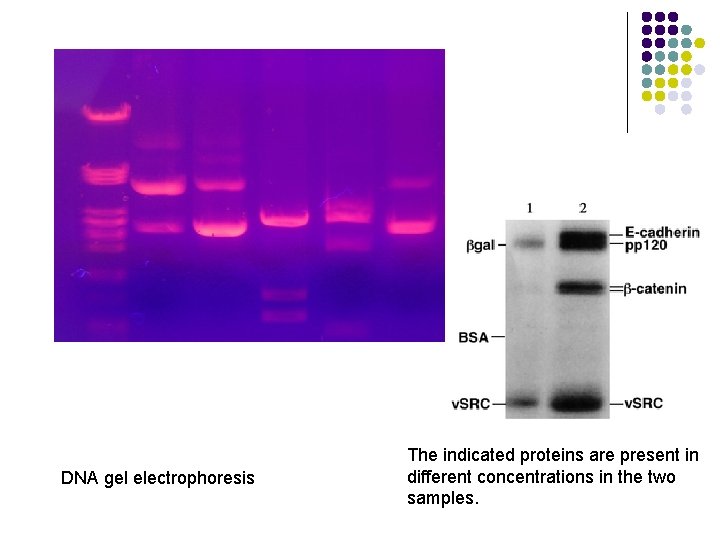
DNA gel electrophoresis The indicated proteins are present in different concentrations in the two samples.

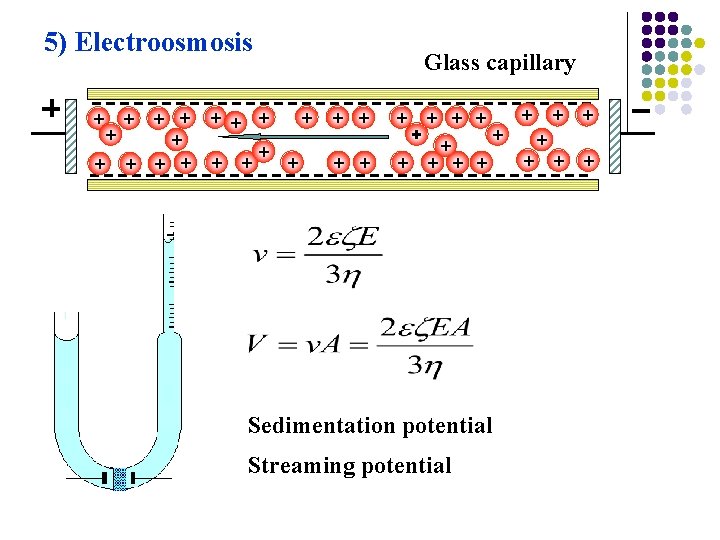
5) Electroosmosis + + + + + Glass capillary + + + + + + Sedimentation potential Streaming potential + + + +