8 3 Temperature and Volume Charless Law If
- Slides: 12

8. 3 Temperature and Volume (Charles’s Law) If we increase the temperature of a gas sample, kinetic molecular theory states that the motion (kinetic energy) of the gas particles will also increase. If the amount and pressure of the gas is held constant, the volume of the container will increase. Learning Goal Use the temperature–volume relationship (Charles’s law) to determine the final temperature or volume when the pressure and amount of gas are constant. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

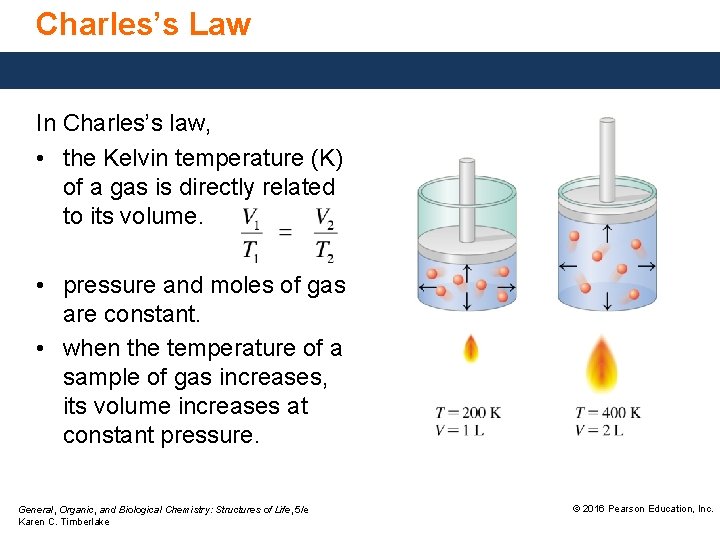
Charles’s Law In Charles’s law, • the Kelvin temperature (K) of a gas is directly related to its volume. • pressure and moles of gas are constant. • when the temperature of a sample of gas increases, its volume increases at constant pressure. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

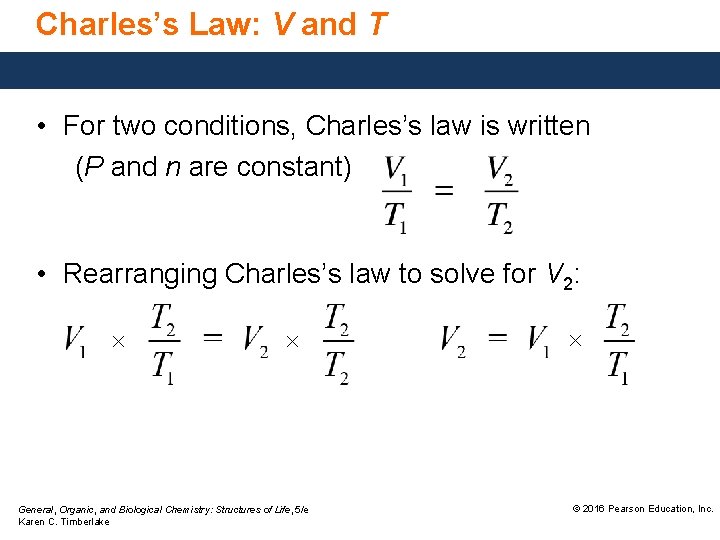
Charles’s Law: V and T • For two conditions, Charles’s law is written (P and n are constant) • Rearranging Charles’s law to solve for V 2: × × General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake × © 2016 Pearson Education, Inc.

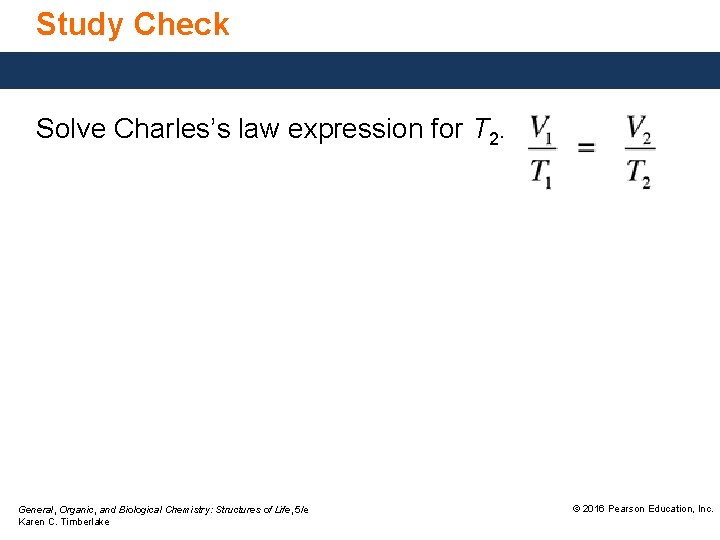
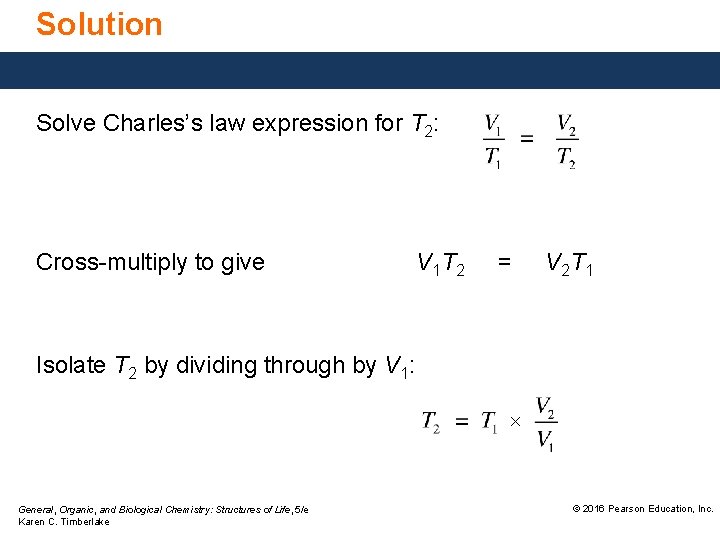
Study Check Solve Charles’s law expression for T 2. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution Solve Charles’s law expression for T 2: Cross multiply to give V 1 T 2 = V 2 T 1 Isolate T 2 by dividing through by V 1: × General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

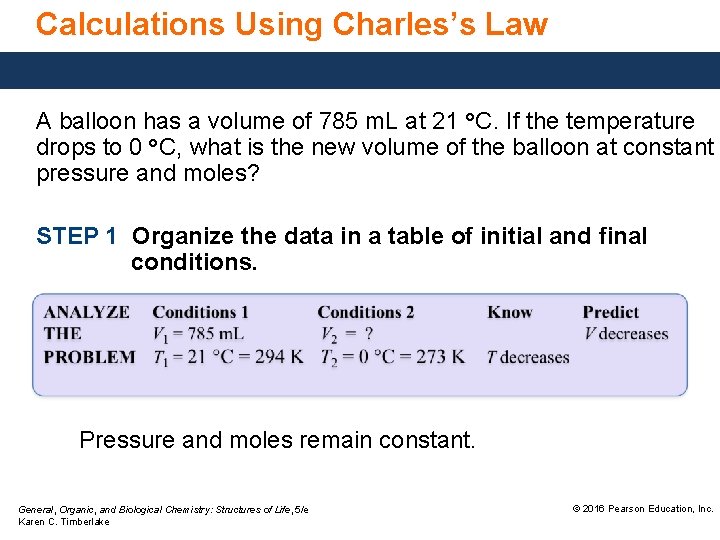
Calculations Using Charles’s Law A balloon has a volume of 785 m. L at 21 °C. If the temperature drops to 0 °C, what is the new volume of the balloon at constant pressure and moles? STEP 1 Organize the data in a table of initial and final conditions. Pressure and moles remain constant. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

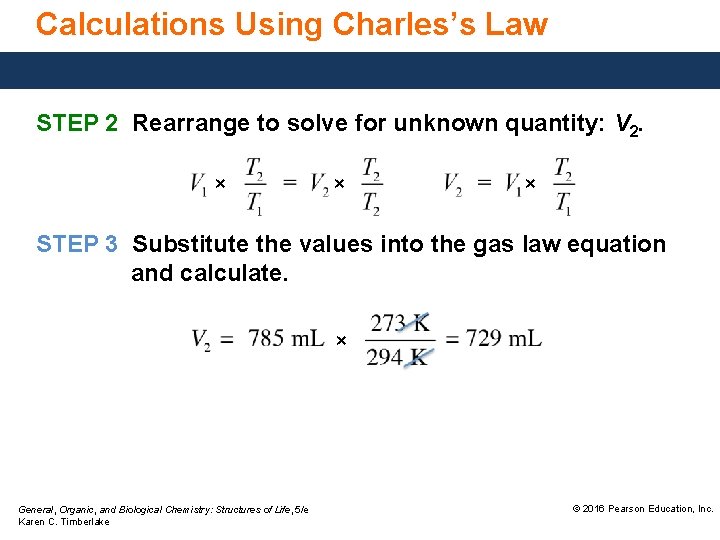
Calculations Using Charles’s Law STEP 2 Rearrange to solve for unknown quantity: V 2. × × × STEP 3 Substitute the values into the gas law equation and calculate. × General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Study Check A sample of oxygen gas has a volume of 420 m. L at a temperature of 18 °C. At what temperature (in °C) will the volume of the oxygen be 640 m. L (P and n are constant)? A. 443 °C B. 170 °C C. − 82 °C General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

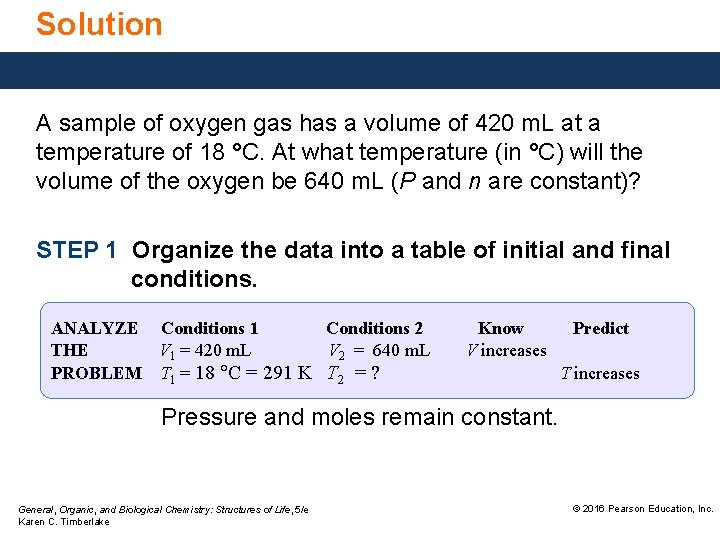
Solution A sample of oxygen gas has a volume of 420 m. L at a temperature of 18 °C. At what temperature (in °C) will the volume of the oxygen be 640 m. L (P and n are constant)? STEP 1 Organize the data into a table of initial and final conditions. ANALYZE Conditions 1 Conditions 2 THE V 1 = 420 m. L V 2 = 640 m. L PROBLEM T 1 = 18 C = 291 K T 2 = ? Know V increases Predict T increases Pressure and moles remain constant. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

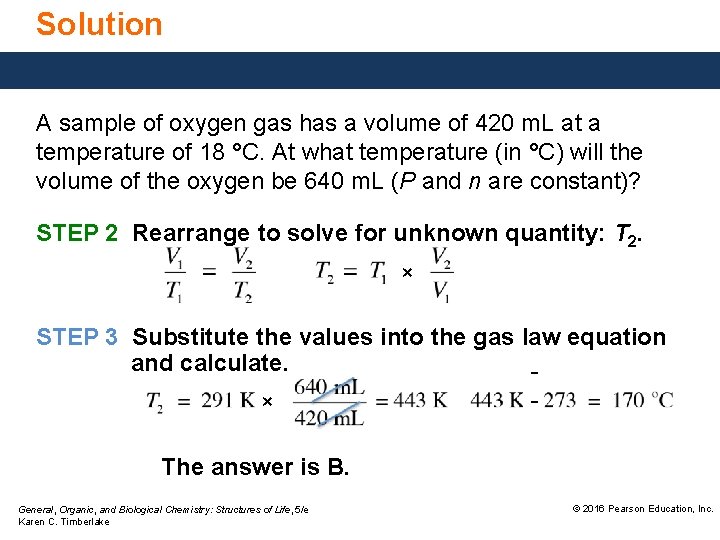
Solution A sample of oxygen gas has a volume of 420 m. L at a temperature of 18 °C. At what temperature (in °C) will the volume of the oxygen be 640 m. L (P and n are constant)? STEP 2 Rearrange to solve for unknown quantity: T 2. × STEP 3 Substitute the values into the gas law equation and calculate. × The answer is B. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

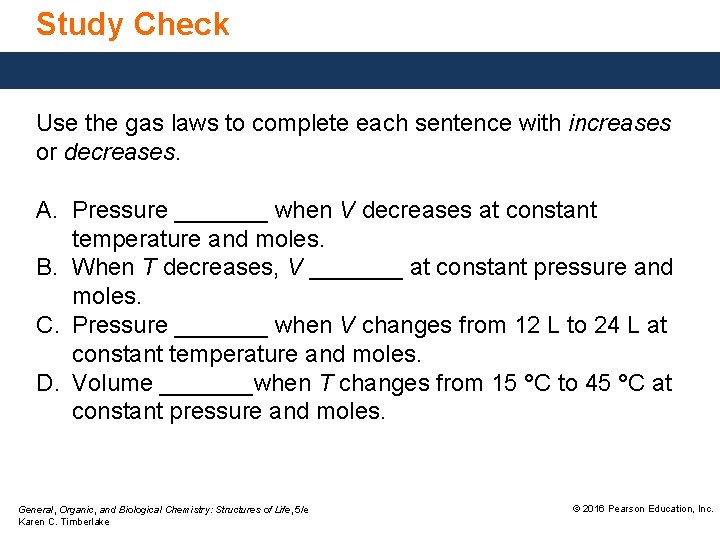
Study Check Use the gas laws to complete each sentence with increases or decreases. A. Pressure _______ when V decreases at constant temperature and moles. B. When T decreases, V _______ at constant pressure and moles. C. Pressure _______ when V changes from 12 L to 24 L at constant temperature and moles. D. Volume _______when T changes from 15 °C to 45 °C at constant pressure and moles. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

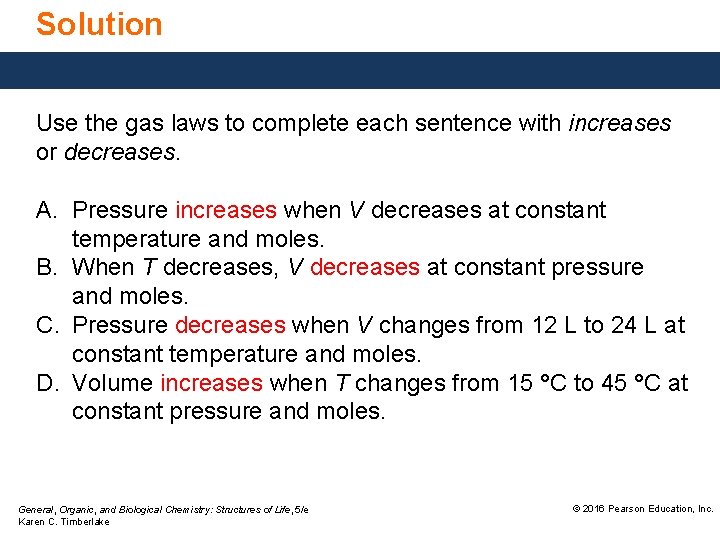
Solution Use the gas laws to complete each sentence with increases or decreases. A. Pressure increases when V decreases at constant temperature and moles. B. When T decreases, V decreases at constant pressure and moles. C. Pressure decreases when V changes from 12 L to 24 L at constant temperature and moles. D. Volume increases when T changes from 15 °C to 45 °C at constant pressure and moles. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.