8 3 Concentration of Solutions Learning Goals use


- Slides: 10

8. 3 Concentration of Solutions Learning Goals … … use various formulae to calculate different types of concentration percentages … use the molar concentration and molar mass formulae to solve for molarity, mass or volume

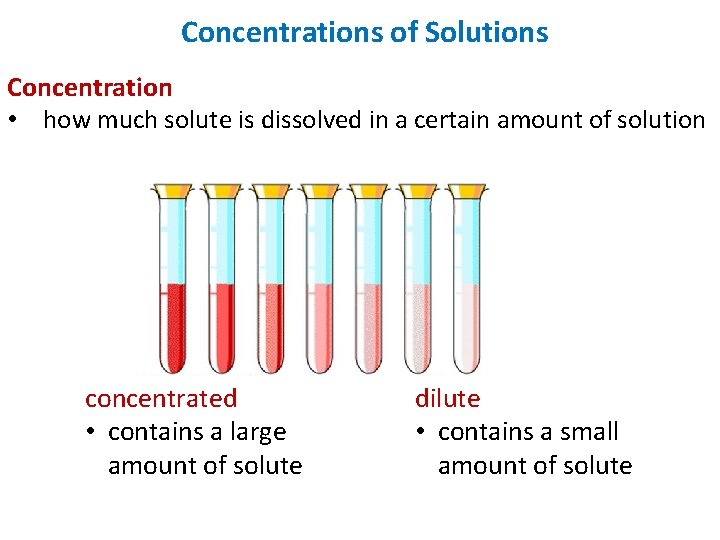
Concentrations of Solutions Concentration • how much solute is dissolved in a certain amount of solution concentrated • contains a large amount of solute dilute • contains a small amount of solute

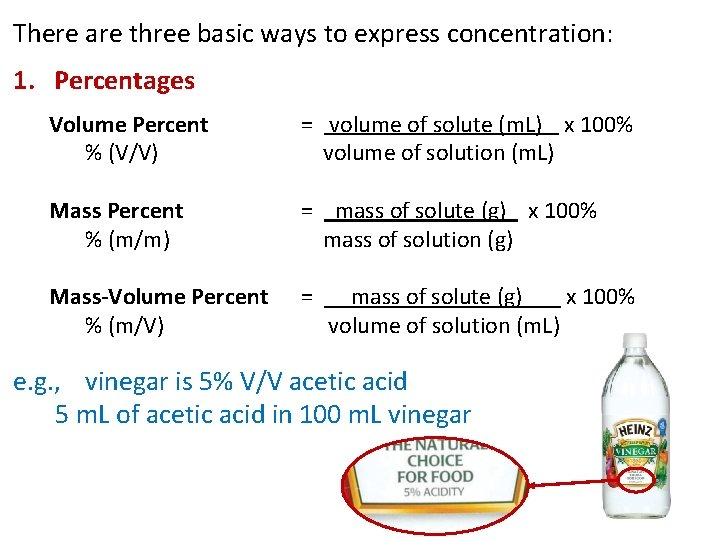
There are three basic ways to express concentration: 1. Percentages Volume Percent % (V/V) = volume of solute (m. L) x 100% volume of solution (m. L) Mass Percent % (m/m) = mass of solute (g) x 100% mass of solution (g) Mass-Volume Percent % (m/V) = mass of solute (g) x 100% volume of solution (m. L) e. g. , vinegar is 5% V/V acetic acid 5 m. L of acetic acid in 100 m. L vinegar

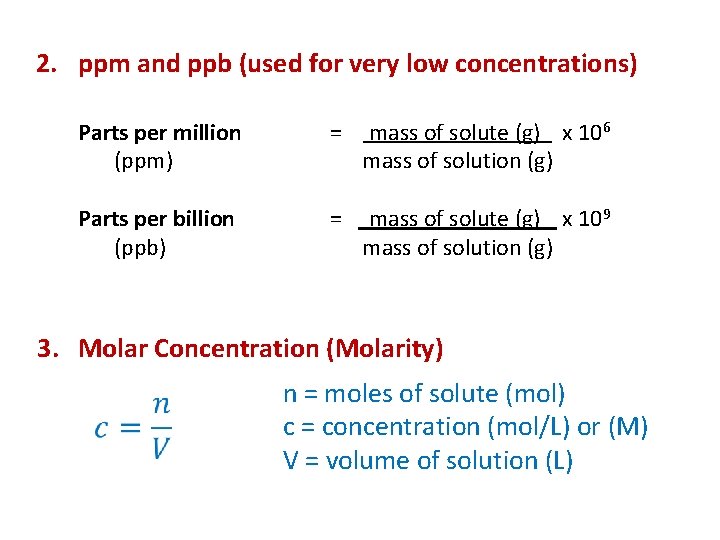
2. ppm and ppb (used for very low concentrations) Parts per million (ppm) = mass of solute (g) x 106 mass of solution (g) Parts per billion (ppb) = mass of solute (g) x 109 mass of solution (g) 3. Molar Concentration (Molarity) n = moles of solute (mol) c = concentration (mol/L) or (M) V = volume of solution (L)

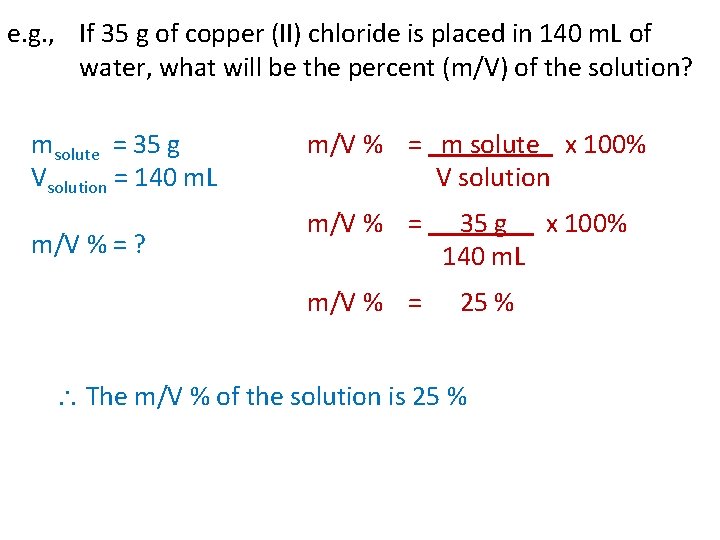
e. g. , If 35 g of copper (II) chloride is placed in 140 m. L of water, what will be the percent (m/V) of the solution? msolute = 35 g Vsolution = 140 m. L m/V % = m solute x 100% V solution m/V % = ? m/V % = 35 g x 100% 140 m. L m/V % = 25 % The m/V % of the solution is 25 %

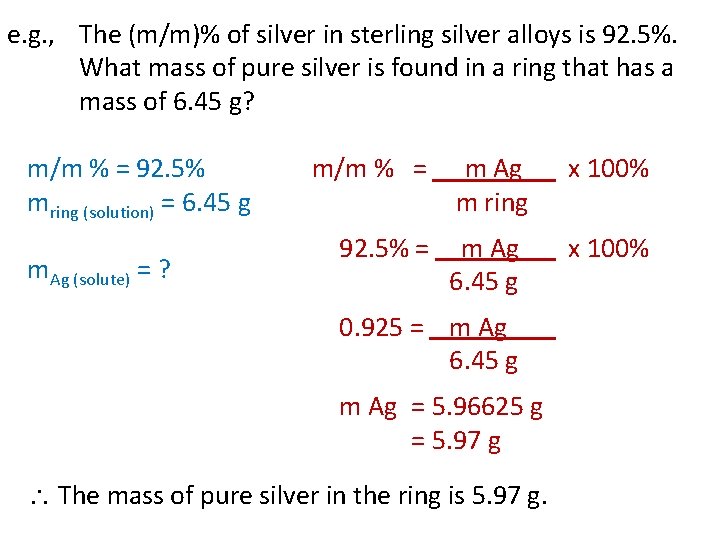
e. g. , The (m/m)% of silver in sterling silver alloys is 92. 5%. What mass of pure silver is found in a ring that has a mass of 6. 45 g? m/m % = 92. 5% m/m % = m Ag x 100% mring (solution) = 6. 45 g m ring m. Ag (solute) = ? 92. 5% = m Ag x 100% 6. 45 g 0. 925 = m Ag 6. 45 g m Ag = 5. 96625 g = 5. 97 g The mass of pure silver in the ring is 5. 97 g.

e. g. , Health Canada’s guideline for the maximum mercury content in commercial fish is 0. 5 ppm. When a 1. 6 kg salmon was tested, it was found to contain 0. 6 mg of mercury. Would this salmon be safe to eat? m. Hg (solute) = 0. 6 mg = 0. 0006 g msalmon (solution) = 1. 6 kg = 1600 g ppm = ? ppm = m Hg (g) x 106 m salmon (g) ppm = 0. 0006 g x 106 1600 g ppm = 0. 375 ppm = 0. 4 ppm the salmon would be safe to eat.

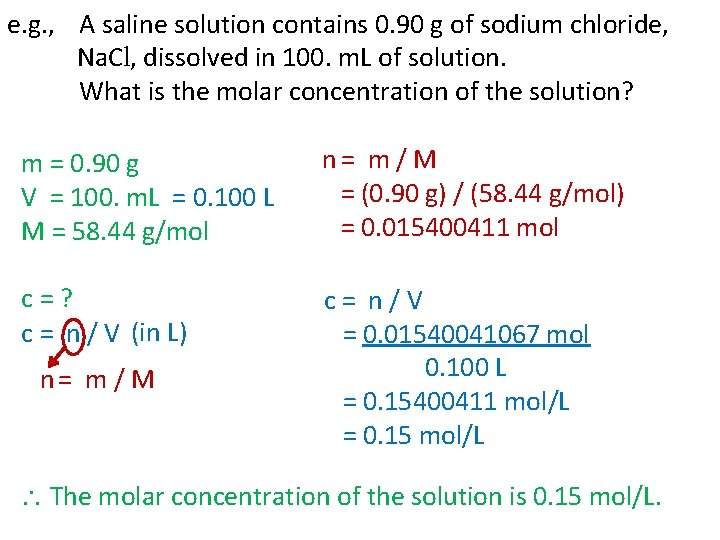
e. g. , A saline solution contains 0. 90 g of sodium chloride, Na. Cl, dissolved in 100. m. L of solution. What is the molar concentration of the solution? m = 0. 90 g V = 100. m. L = 0. 100 L M = 58. 44 g/mol n = m / M = (0. 90 g) / (58. 44 g/mol) = 0. 015400411 mol c = ? c = n / V (in L) c = n / V = 0. 01540041067 mol 0. 100 L = 0. 15400411 mol/L = 0. 15 mol/L n = m / M The molar concentration of the solution is 0. 15 mol/L.

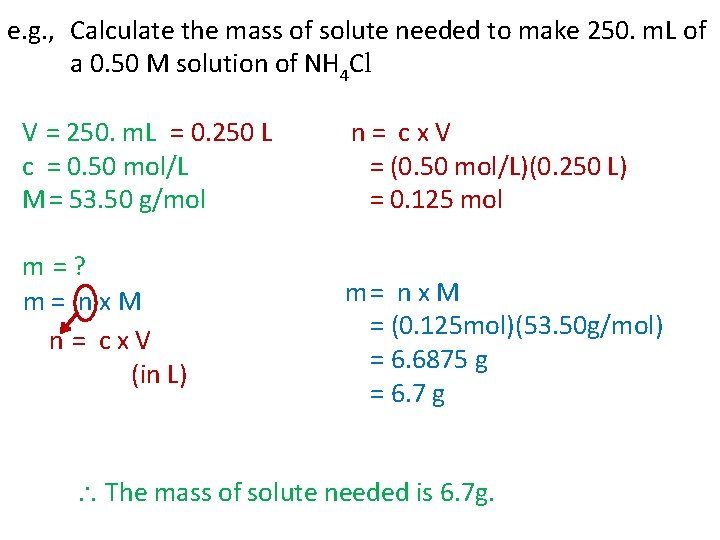
e. g. , Calculate the mass of solute needed to make 250. m. L of a 0. 50 M solution of NH 4 Cl V = 250. m. L = 0. 250 L c = 0. 50 mol/L M = 53. 50 g/mol m = ? m = n x M n = c x V (in L) n = c x V = (0. 50 mol/L)(0. 250 L) = 0. 125 mol m = n x M = (0. 125 mol)(53. 50 g/mol) = 6. 6875 g = 6. 7 g The mass of solute needed is 6. 7 g.

CAN I … … use various formulae to calculate different types of concentration percentages? … use the molar concentration and molar mass formulae to solve for molarity, mass, or volume? HOMEWORK p 373 #6, 7 p 375 #15, 16 p 376 #25 p 378 #33, 34 p 381 #45 ac, 47 -50