8 1 Molecular Compounds Chapter 8 Covalent Bonding

8. 1 Molecular Compounds > Chapter 8 Covalent Bonding 8. 1 Molecular Compounds 8. 2 The Nature of Covalent Bonding 8. 3 Bonding Theories 8. 4 Polar Bonds and Molecules 1 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

8. 1 Molecular Compounds > Do Now 1. Write down one goal you have for yourself in this class for Marking Period 2. 2. Fill out the Ionic Compound column of the graphic organizer. 2 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
8. 1 Molecular Compounds > Do Now Write the formula of the ionic compound formed when the following elements combine: a) b) c) d) e) 3 Sodium and Chlorine Lithium and Oxygen Magnesium and Fluorine Calcium and Sulfur Potassium and Nitrogen Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
8. 1 Molecular Compounds > Homework Policy • Late assignments will result in a grade reduction of 10 pts per day. • 2 missed assignments will result in parental notification. 4 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
8. 1 Molecular Compounds > Molecules and Molecular Compounds In nature, only the noble gas elements exist as uncombined atoms. • They are monatomic; that is, they consist of single atoms. • Helium, which is less dense than air, is often used to inflate balloons. 5 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
8. 1 Molecular Compounds > Molecules and Molecular Compounds There are two ways elements can be like the nearest noble gas. 1. Transfer electrons to form an ionic compound. 2. Share electrons to form covalent compounds. 6 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
8. 1 Molecular Compounds > Molecules and Molecular Compounds Use the element cards and chips to model the bonding of 2 fluorine atoms. Each chip is one valence electron. How can they share electrons to achieve the electron configurations of a noble gas? 7 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
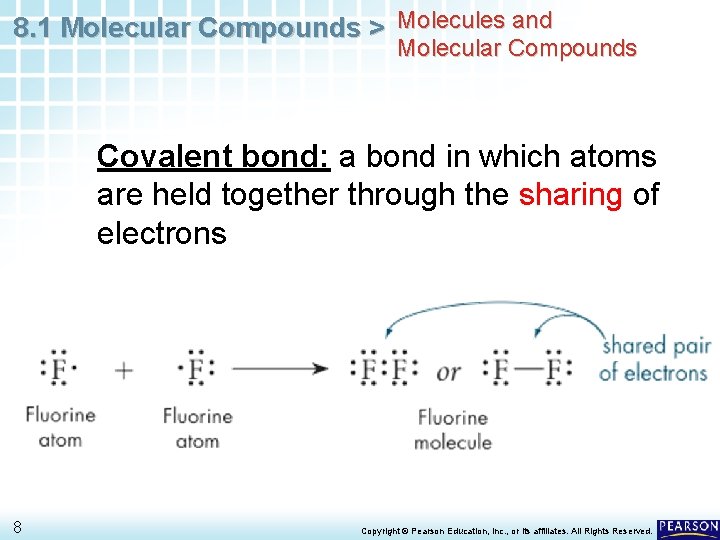
8. 1 Molecular Compounds > Molecules and Molecular Compounds Covalent bond: a bond in which atoms are held together through the sharing of electrons 8 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
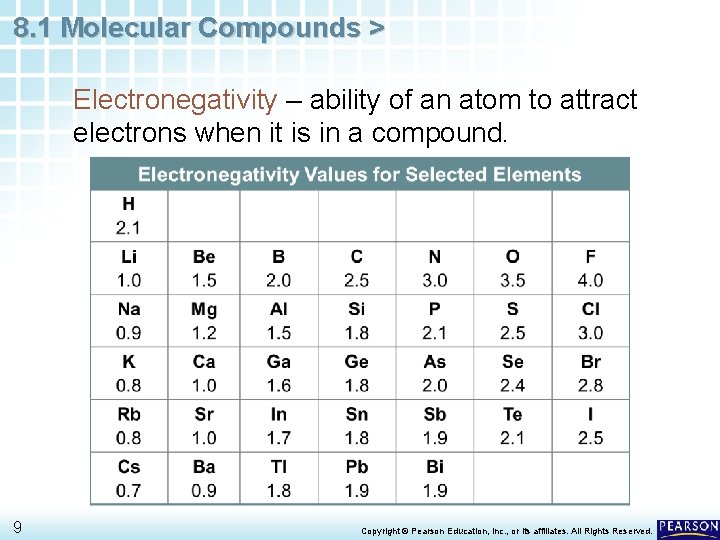
8. 1 Molecular Compounds > Electronegativity – ability of an atom to attract electrons when it is in a compound. 9 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
8. 1 Molecular Compounds > Molecules and Molecular Compounds The electronegativity difference between two atoms tells you what kind of bond is likely to form. >2. 0 tends to form an ionic bond <2 tends to form a covalent bond 10 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
8. 1 Molecular Compounds > Molecules and Molecular Compounds Molecule: a neutral group of atoms joined together by covalent bonds Examples: F 2 H 2 O CH 4 11 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
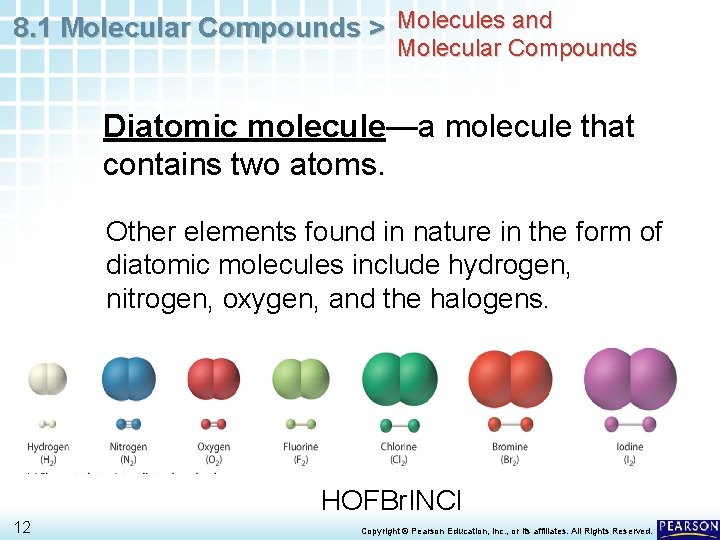
8. 1 Molecular Compounds > Molecules and Molecular Compounds Diatomic molecule—a molecule that contains two atoms. Other elements found in nature in the form of diatomic molecules include hydrogen, nitrogen, oxygen, and the halogens. HOFBr. INCl 12 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
8. 1 Molecular Compounds > Molecules and Molecular Compounds Draw the electron dot structures for the following molecules. a. Chlorine, Cl 2 b. Bromine, Br 2 c. Iodine, I 2 13 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
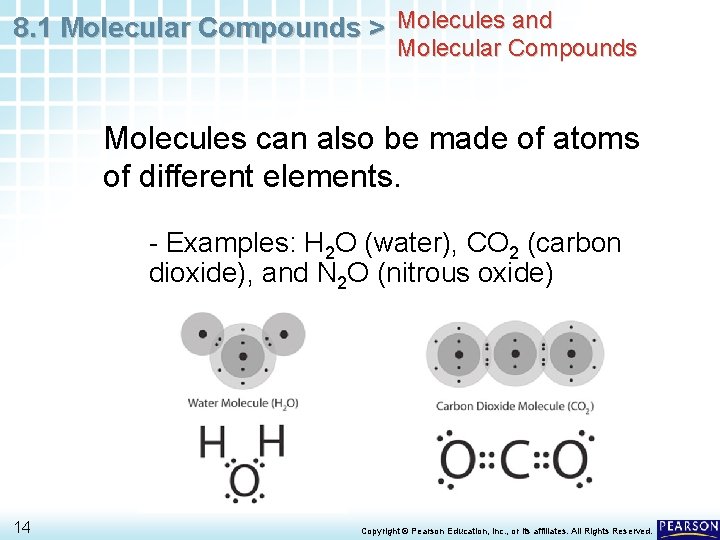
8. 1 Molecular Compounds > Molecules and Molecular Compounds Molecules can also be made of atoms of different elements. - Examples: H 2 O (water), CO 2 (carbon dioxide), and N 2 O (nitrous oxide) 14 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
8. 1 Molecular Compounds > Molecules and Molecular Compounds Molecular formula - chemical formula of a molecular compound. • shows how many atoms of each element a substance contains H 2 O – 2 Hydrogen, 1 Oxygen C 4 H 10 – 4 Carbon, 10 Hydrogen 15 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
8. 1 Molecular Compounds > Molecules and Molecular Compounds Identify the number and kinds of atoms present in a molecule of each compound. C 6 H 8 O 6 – ascorbic acid (vitamin C) C 12 H 22 O 11 – sucrose (table sugar) C 7 H 5 N 3 O 6 – trinitrotoluene (TNT) 16 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
8. 1 Molecular Compounds > Comparing Molecular and Ionic Compounds Properties of Molecular Compounds • Lower melting and boiling points than ionic compounds • Solids, liquids or gases at room temperature • Composed of atoms of two or more nonmetals • Do not conduct electricity • Do not dissolve in water as well as ionic compounds 17 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
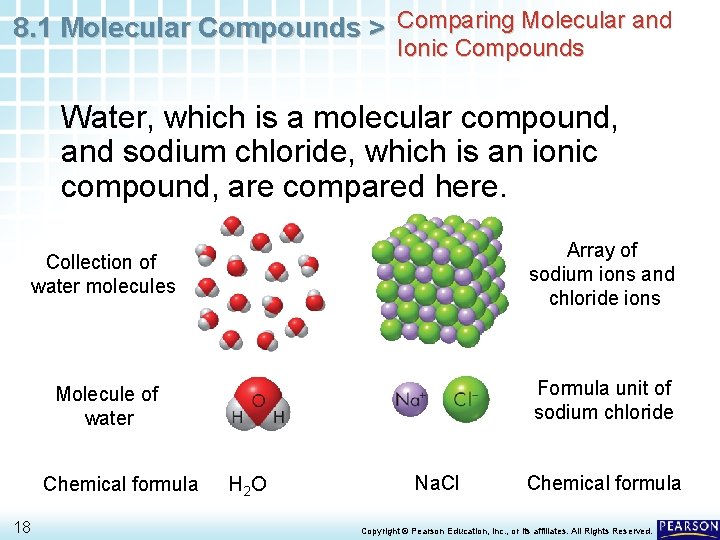
8. 1 Molecular Compounds > Comparing Molecular and Ionic Compounds Water, which is a molecular compound, and sodium chloride, which is an ionic compound, are compared here. Collection of water molecules Array of sodium ions and chloride ions Molecule of water Formula unit of sodium chloride Chemical formula 18 H 2 O Na. Cl Chemical formula Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

8. 1 Molecular Compounds > Molecules and Molecular Compounds Classify compounds into groups according to their chemical bonding (ionic or covalent). Sodium Chloride, Na. Cl Methane, CH 4 Oxygen, O 2 Ammonia, NH 3 Magnesium Oxide, Mg. O Barium Iodide, Ba. I 2 High or low melting point? Conduct electricity? 19 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
8. 1 Molecular Compounds > END OF 8. 1 20 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
- Slides: 20