7 The Mole Number of moles mass in

7 The Mole
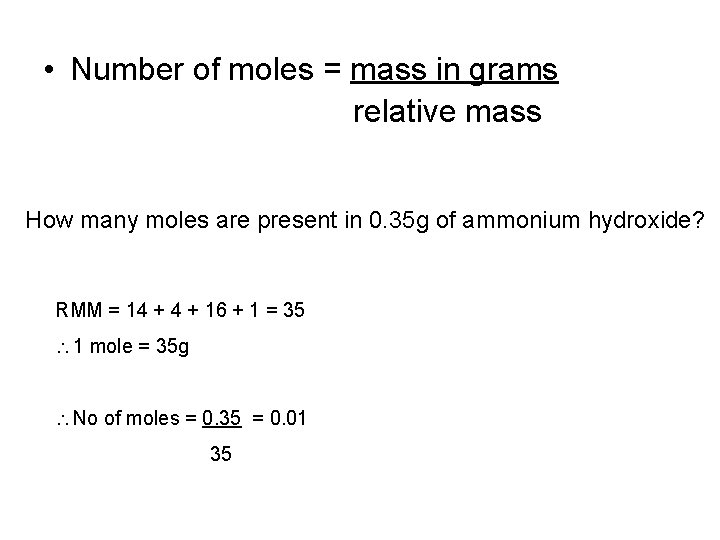
• Number of moles = mass in grams relative mass How many moles are present in 0. 35 g of ammonium hydroxide? RMM = 14 + 16 + 1 = 35 1 mole = 35 g No of moles = 0. 35 = 0. 01 35

Find the mass of a) 1 mole of lead (II) nitrate b) 4. 30 moles of methane c) 0. 24 moles of hydrated sodium carbonate Na 2 CO 3. 10 H 2 O
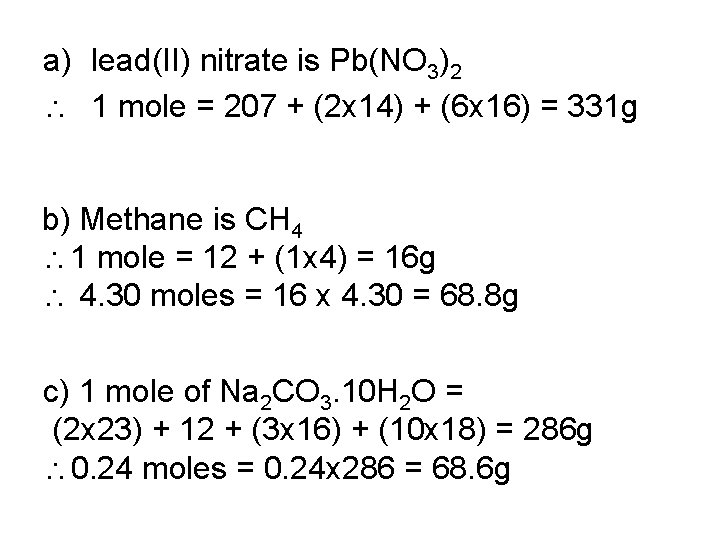
a) lead(II) nitrate is Pb(NO 3)2 1 mole = 207 + (2 x 14) + (6 x 16) = 331 g b) Methane is CH 4 1 mole = 12 + (1 x 4) = 16 g 4. 30 moles = 16 x 4. 30 = 68. 8 g c) 1 mole of Na 2 CO 3. 10 H 2 O = (2 x 23) + 12 + (3 x 16) + (10 x 18) = 286 g 0. 24 moles = 0. 24 x 286 = 68. 6 g

• Avogadros’s no = 6. 022 x 1023 • It is the number of atoms in 12 g of 12 C • The RAM of an element contains Avogadros number of atoms i. e if we weigh out 12 g of carbon-12 we will have 6. 022 x 1023 atoms of carbon-12 and 12 g of carbon-12 = 1 mole
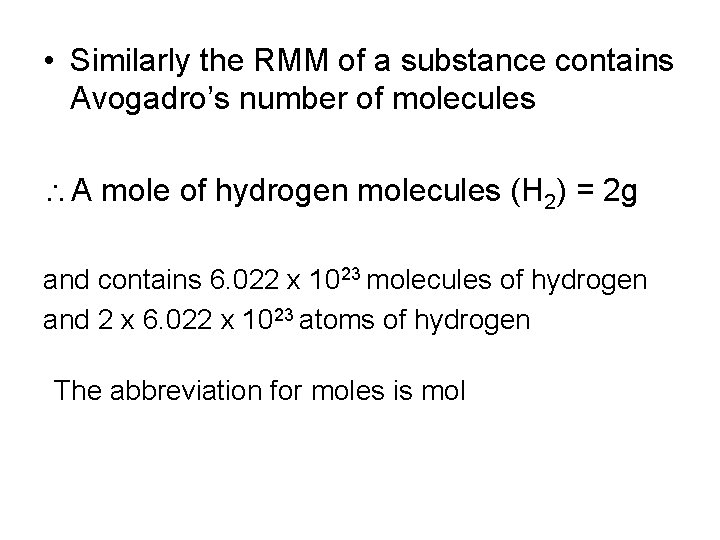
• Similarly the RMM of a substance contains Avogadro’s number of molecules A mole of hydrogen molecules (H 2) = 2 g and contains 6. 022 x 1023 molecules of hydrogen and 2 x 6. 022 x 1023 atoms of hydrogen The abbreviation for moles is mol

A mole of substance is the amount of that substance that contains the same number of stated elementary units as there atoms in 12 g of 12 C Stated elementary units can mean atoms, molecules ions, electrons

For example 16 g (1 mole) of oxygen atoms O 32 g (1 mole) of oxygen molecules O 2 18 g (1 mole) of water molecules H 2 O 24 g of magnesium ions Mg 2+

Which of the following contains the greatest number of the stated particles? a) Molecules of hydrogen in 1 g of hydrogen gas b) Atoms of helium in 1 g of helium gas c) Atoms of beryllium in 1 g of beryllium

1 g of hydrogen gas = 1 mol = 0. 5 mol 2 1 g hydrogen gas contains 0. 5 x 6. 022 x 1023 molecules = 3. 011 x 1023 molecules 1 g of helium gas contains 1 mol = 0. 25 mol 4 1 g helium gas contains 0. 25 x 6. 022 x 1023 atoms = 1. 51 x 1023 atoms 1 g beryllium contains 1 mol = 0. 11 mol 9 1 g beryllium contains 0. 11 x 6. 022 x 1023 atoms = 6. 69 x 1022 atoms 1 g hydrogen gas contains the greatest number of the stated particles
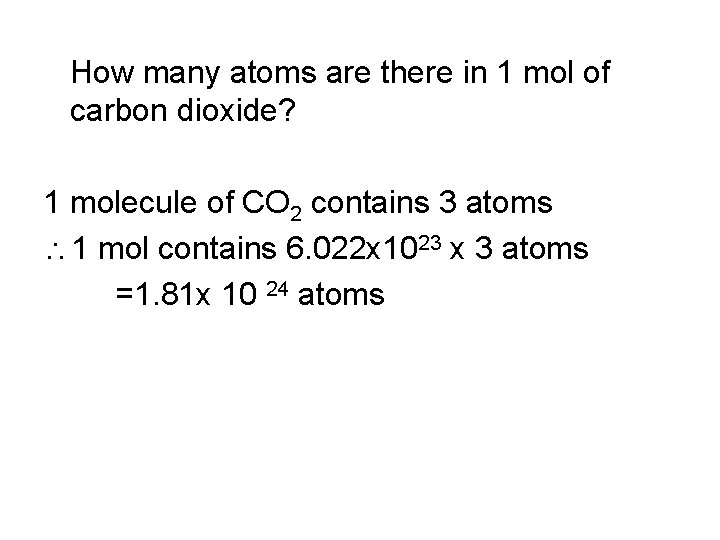
How many atoms are there in 1 mol of carbon dioxide? 1 molecule of CO 2 contains 3 atoms 1 mol contains 6. 022 x 1023 x 3 atoms =1. 81 x 10 24 atoms
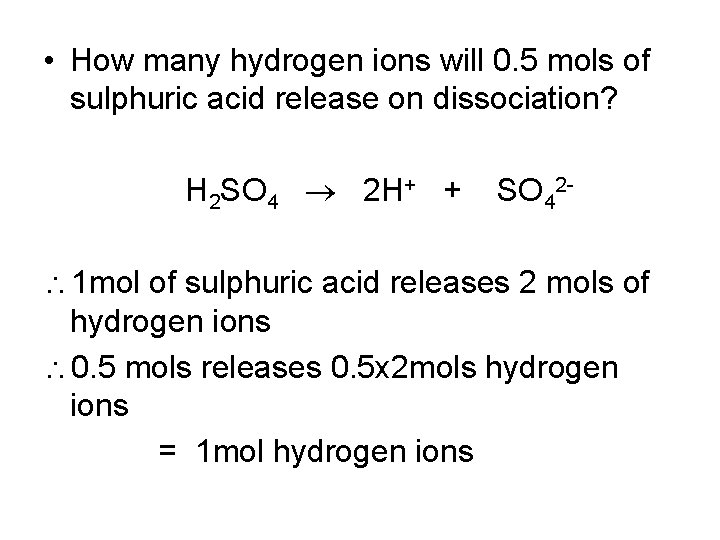
• How many hydrogen ions will 0. 5 mols of sulphuric acid release on dissociation? H 2 SO 4 2 H+ + SO 42 - 1 mol of sulphuric acid releases 2 mols of hydrogen ions 0. 5 mols releases 0. 5 x 2 mols hydrogen ions = 1 mol hydrogen ions
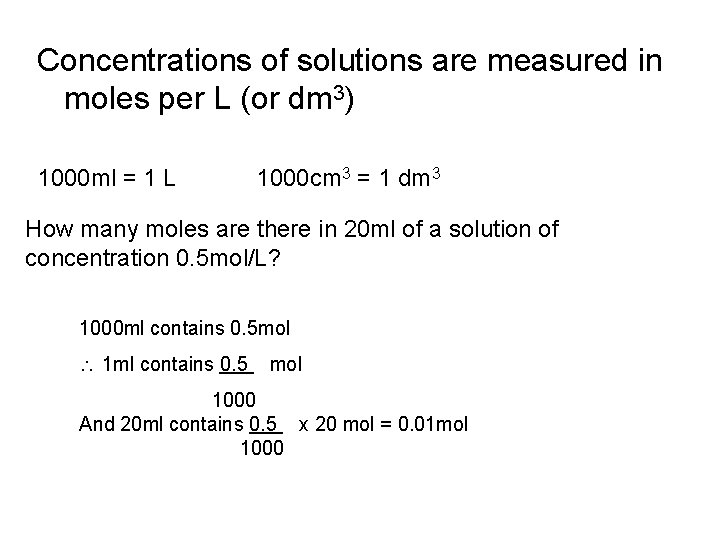
Concentrations of solutions are measured in moles per L (or dm 3) 1000 ml = 1 L 1000 cm 3 = 1 dm 3 How many moles are there in 20 ml of a solution of concentration 0. 5 mol/L? 1000 ml contains 0. 5 mol 1 ml contains 0. 5 mol 1000 And 20 ml contains 0. 5 x 20 mol = 0. 01 mol 1000
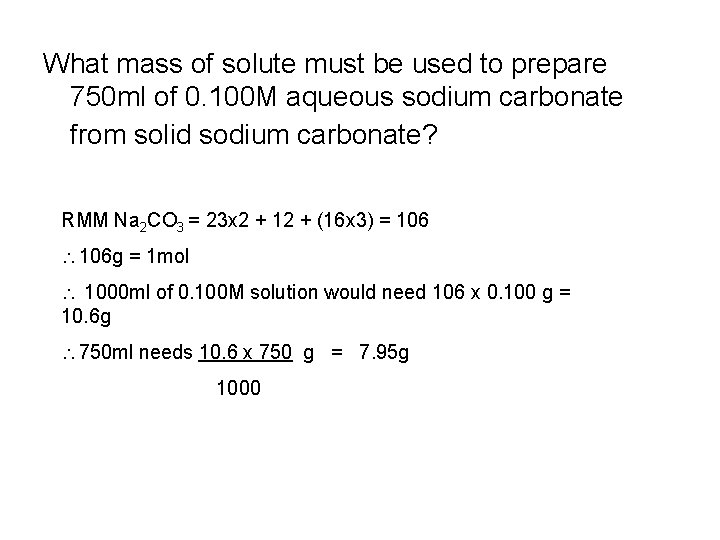
What mass of solute must be used to prepare 750 ml of 0. 100 M aqueous sodium carbonate from solid sodium carbonate? RMM Na 2 CO 3 = 23 x 2 + 12 + (16 x 3) = 106 g = 1 mol 1000 ml of 0. 100 M solution would need 106 x 0. 100 g = 10. 6 g 750 ml needs 10. 6 x 750 g = 7. 95 g 1000
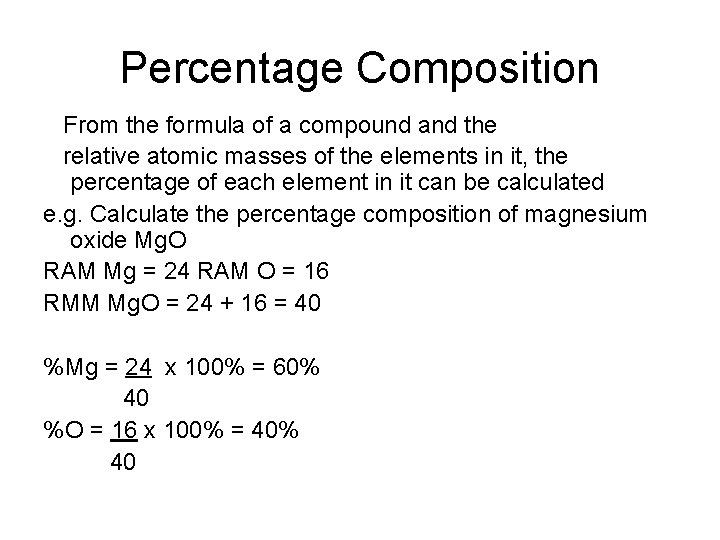
Percentage Composition From the formula of a compound and the relative atomic masses of the elements in it, the percentage of each element in it can be calculated e. g. Calculate the percentage composition of magnesium oxide Mg. O RAM Mg = 24 RAM O = 16 RMM Mg. O = 24 + 16 = 40 %Mg = 24 x 100% = 60% 40 %O = 16 x 100% = 40% 40
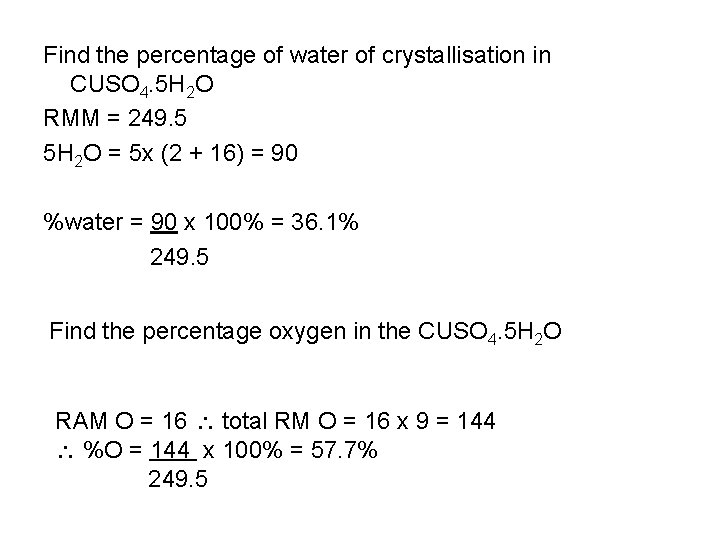
Find the percentage of water of crystallisation in CUSO 4. 5 H 2 O RMM = 249. 5 5 H 2 O = 5 x (2 + 16) = 90 %water = 90 x 100% = 36. 1% 249. 5 Find the percentage oxygen in the CUSO 4. 5 H 2 O RAM O = 16 total RM O = 16 x 9 = 144 %O = 144 x 100% = 57. 7% 249. 5
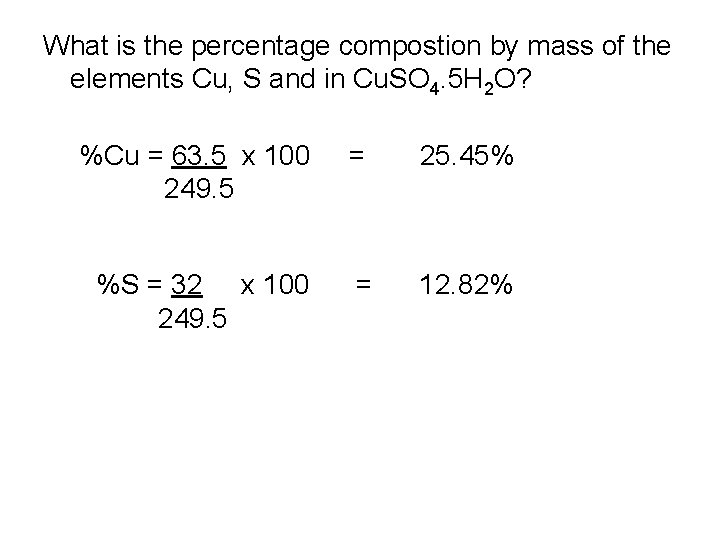
What is the percentage compostion by mass of the elements Cu, S and in Cu. SO 4. 5 H 2 O? %Cu = 63. 5 x 100 249. 5 = 25. 45% %S = 32 x 100 249. 5 = 12. 82%
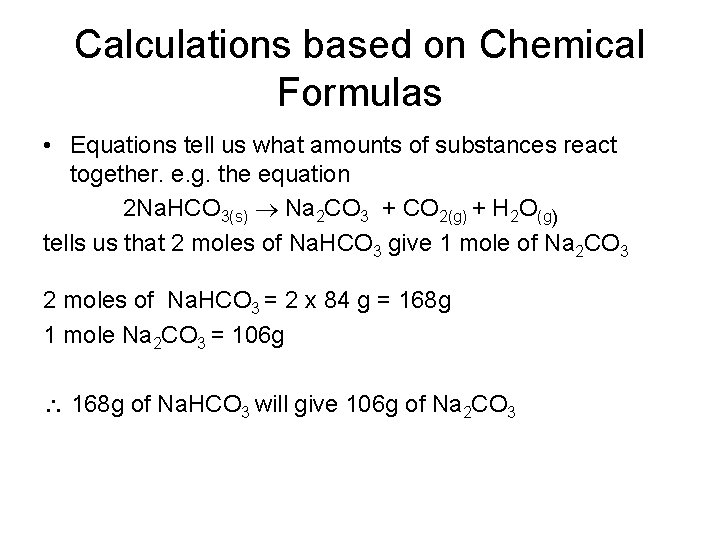
Calculations based on Chemical Formulas • Equations tell us what amounts of substances react together. e. g. the equation 2 Na. HCO 3(s) Na 2 CO 3 + CO 2(g) + H 2 O(g) tells us that 2 moles of Na. HCO 3 give 1 mole of Na 2 CO 3 2 moles of Na. HCO 3 = 2 x 84 g = 168 g 1 mole Na 2 CO 3 = 106 g 168 g of Na. HCO 3 will give 106 g of Na 2 CO 3

The amounts of substances undergoing reaction, as given by the balanced equation, are called the stoichiometric amounts Stoichiometry is the relationship between the amounts of reactants and products in a chemical reaction If one reactant is present in excess of the stoichiometric amount required for the reaction with another of the reactants, then the excess of one reactant will be left unused at the end of the reaction.
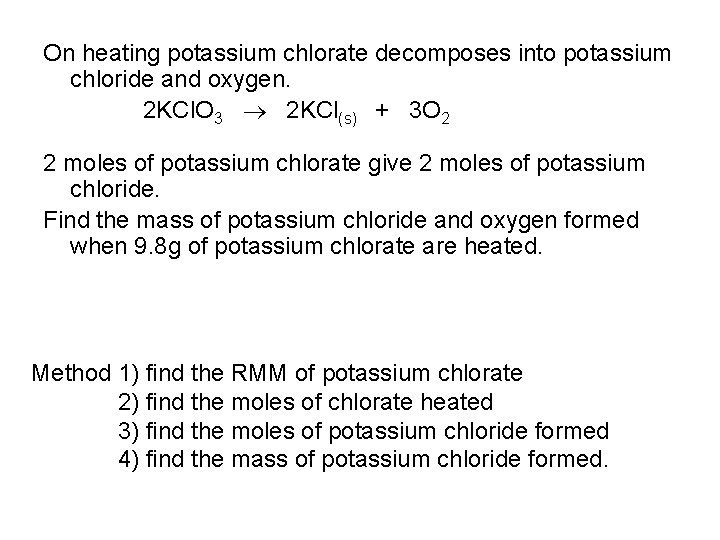
On heating potassium chlorate decomposes into potassium chloride and oxygen. 2 KCl. O 3 2 KCl(s) + 3 O 2 2 moles of potassium chlorate give 2 moles of potassium chloride. Find the mass of potassium chloride and oxygen formed when 9. 8 g of potassium chlorate are heated. Method 1) find the RMM of potassium chlorate 2) find the moles of chlorate heated 3) find the moles of potassium chloride formed 4) find the mass of potassium chloride formed.
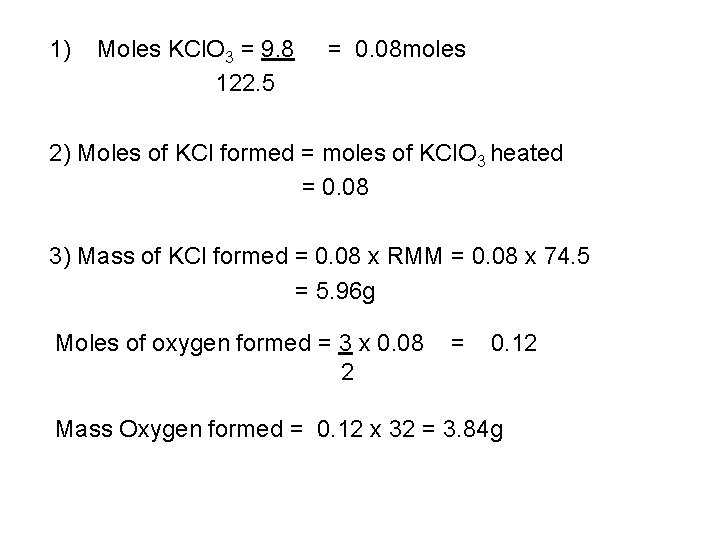
1) Moles KCl. O 3 = 9. 8 122. 5 = 0. 08 moles 2) Moles of KCl formed = moles of KCl. O 3 heated = 0. 08 3) Mass of KCl formed = 0. 08 x RMM = 0. 08 x 74. 5 = 5. 96 g Moles of oxygen formed = 3 x 0. 08 2 = 0. 12 Mass Oxygen formed = 0. 12 x 32 = 3. 84 g
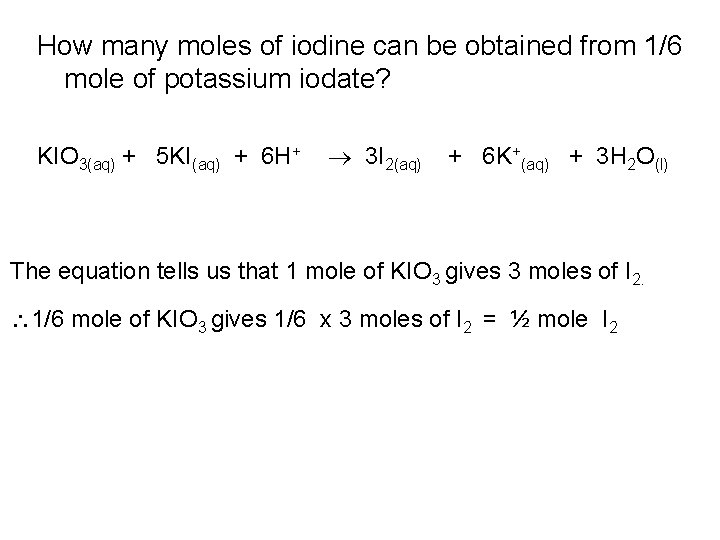
How many moles of iodine can be obtained from 1/6 mole of potassium iodate? KIO 3(aq) + 5 KI(aq) + 6 H+ 3 I 2(aq) + 6 K+(aq) + 3 H 2 O(l) The equation tells us that 1 mole of KIO 3 gives 3 moles of I 2. 1/6 mole of KIO 3 gives 1/6 x 3 moles of I 2 = ½ mole I 2
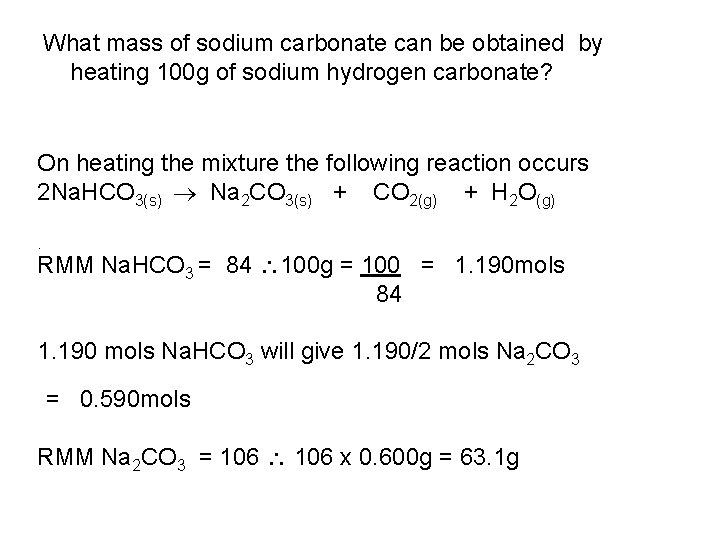
What mass of sodium carbonate can be obtained by heating 100 g of sodium hydrogen carbonate? On heating the mixture the following reaction occurs 2 Na. HCO 3(s) Na 2 CO 3(s) + CO 2(g) + H 2 O(g). RMM Na. HCO 3 = 84 100 g = 100 = 1. 190 mols 84 1. 190 mols Na. HCO 3 will give 1. 190/2 mols Na 2 CO 3 = 0. 590 mols RMM Na 2 CO 3 = 106 x 0. 600 g = 63. 1 g
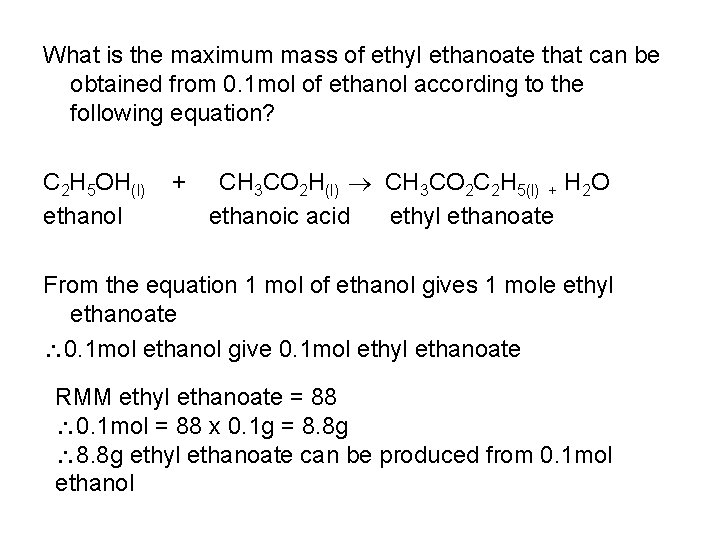
What is the maximum mass of ethyl ethanoate that can be obtained from 0. 1 mol of ethanol according to the following equation? C 2 H 5 OH(l) ethanol + CH 3 CO 2 H(l) CH 3 CO 2 C 2 H 5(l) + H 2 O ethanoic acid ethyl ethanoate From the equation 1 mol of ethanol gives 1 mole ethyl ethanoate 0. 1 mol ethanol give 0. 1 mol ethyl ethanoate RMM ethyl ethanoate = 88 0. 1 mol = 88 x 0. 1 g = 8. 8 g ethyl ethanoate can be produced from 0. 1 mol ethanol
- Slides: 24