7 Angular Momentum 7 A Angular Momentum Commutation



- Slides: 37

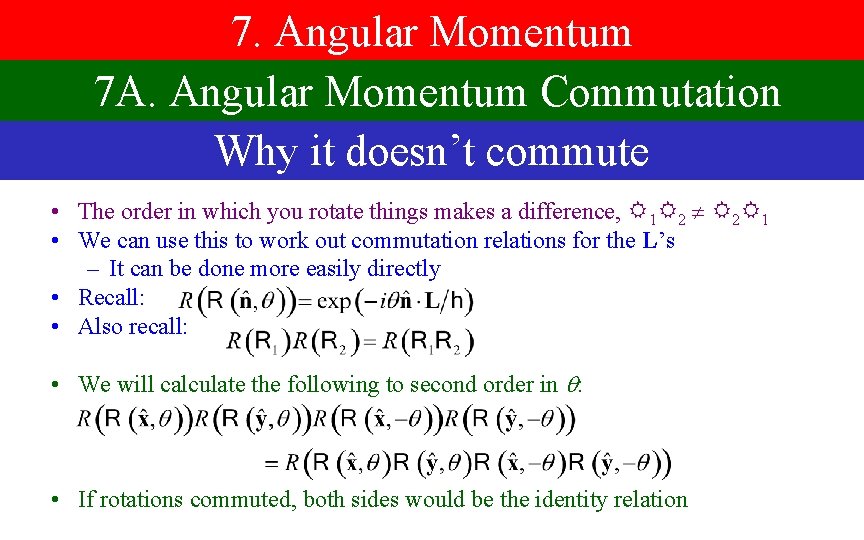
7. Angular Momentum 7 A. Angular Momentum Commutation Why it doesn’t commute • The order in which you rotate things makes a difference, 1 2 2 1 • We can use this to work out commutation relations for the L’s – It can be done more easily directly • Recall: • Also recall: • We will calculate the following to second order in : • If rotations commuted, both sides would be the identity relation

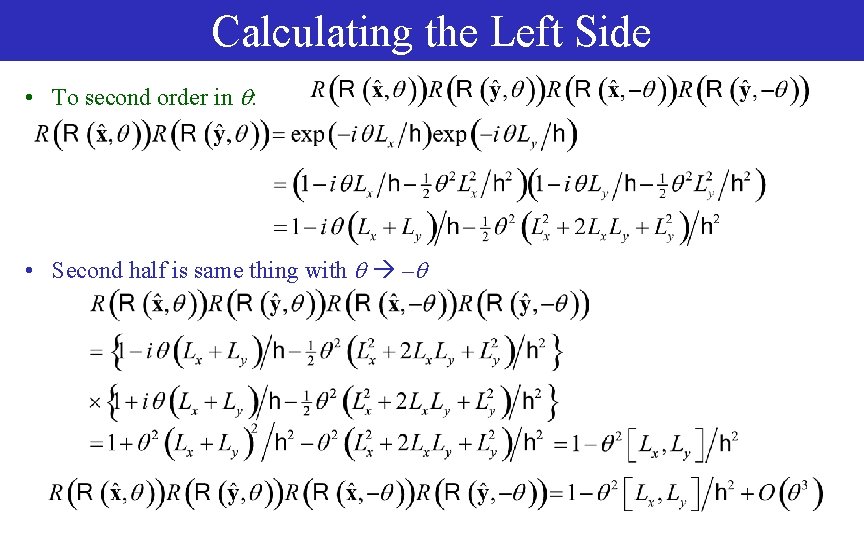
Calculating the Left Side • To second order in : • Second half is same thing with –

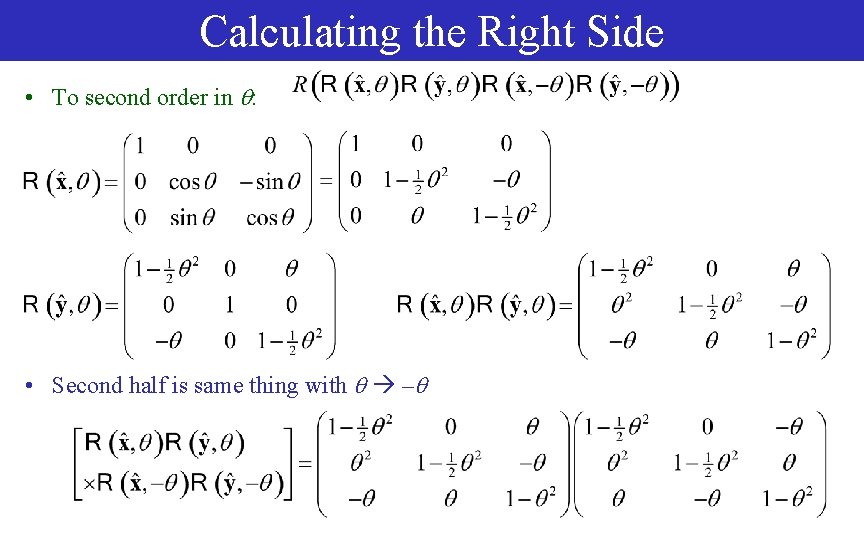
Calculating the Right Side • To second order in : • Second half is same thing with –

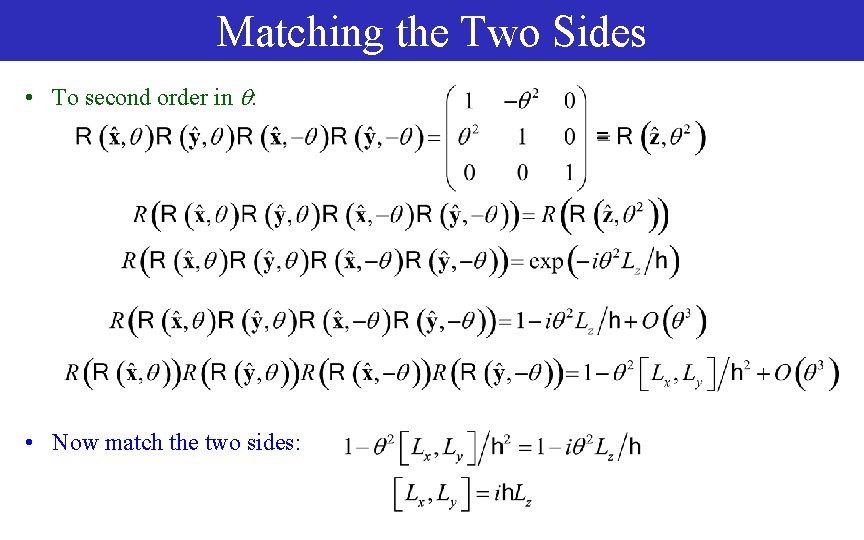
Matching the Two Sides • To second order in : • Now match the two sides:

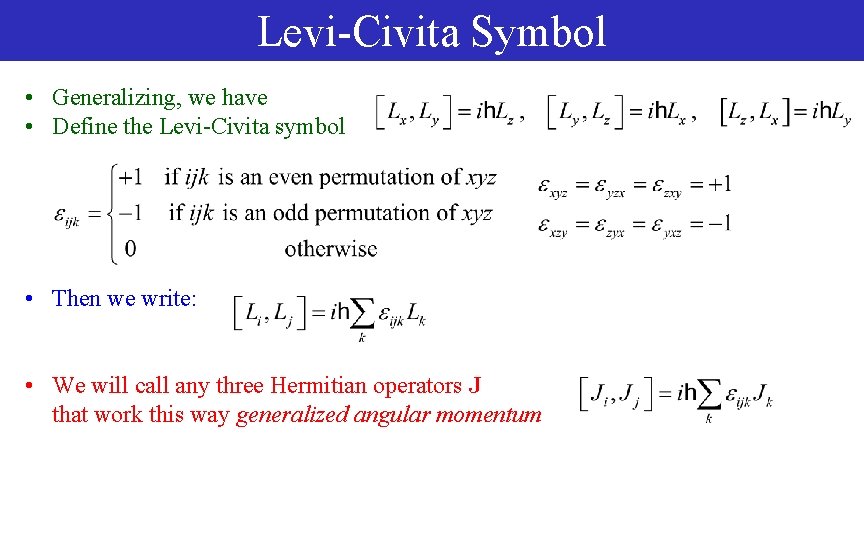
Levi-Civita Symbol • Generalizing, we have • Define the Levi-Civita symbol • Then we write: • We will call any three Hermitian operators J that work this way generalized angular momentum

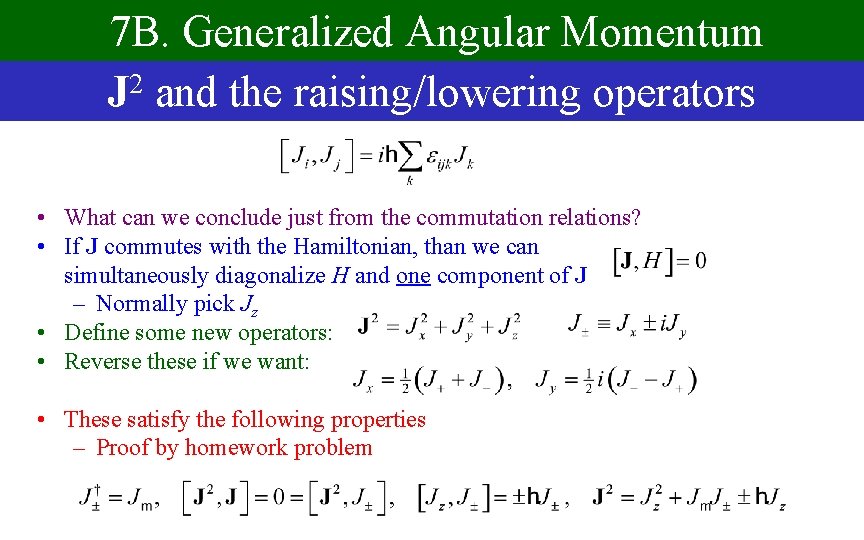
7 B. Generalized Angular Momentum J 2 and the raising/lowering operators • What can we conclude just from the commutation relations? • If J commutes with the Hamiltonian, than we can simultaneously diagonalize H and one component of J – Normally pick Jz • Define some new operators: • Reverse these if we want: • These satisfy the following properties – Proof by homework problem

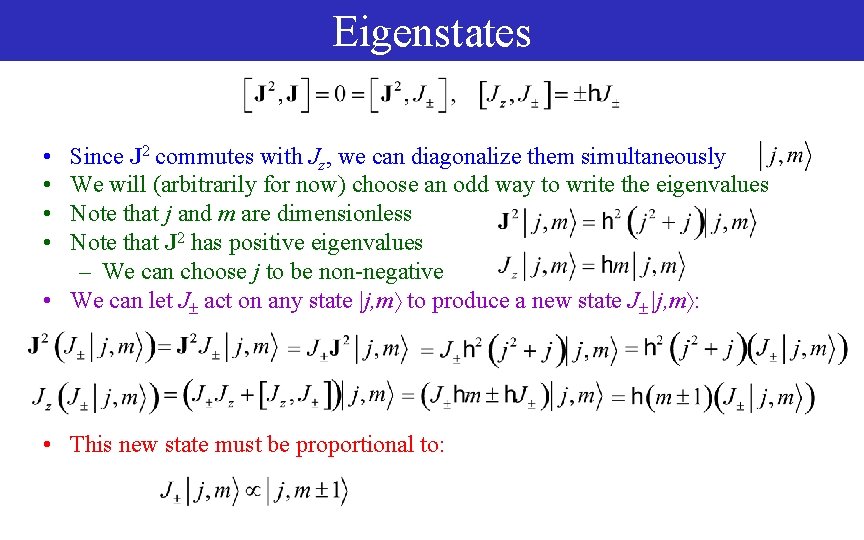
Eigenstates • • Since J 2 commutes with Jz, we can diagonalize them simultaneously We will (arbitrarily for now) choose an odd way to write the eigenvalues Note that j and m are dimensionless Note that J 2 has positive eigenvalues – We can choose j to be non-negative • We can let J act on any state |j, m to produce a new state J |j, m : • This new state must be proportional to:

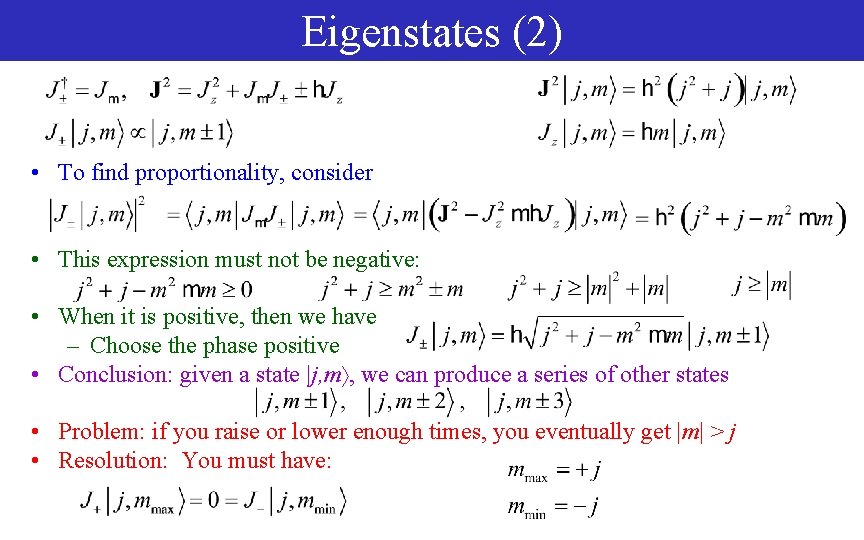
Eigenstates (2) • To find proportionality, consider • This expression must not be negative: • When it is positive, then we have – Choose the phase positive • Conclusion: given a state |j, m , we can produce a series of other states • Problem: if you raise or lower enough times, you eventually get |m| > j • Resolution: You must have:

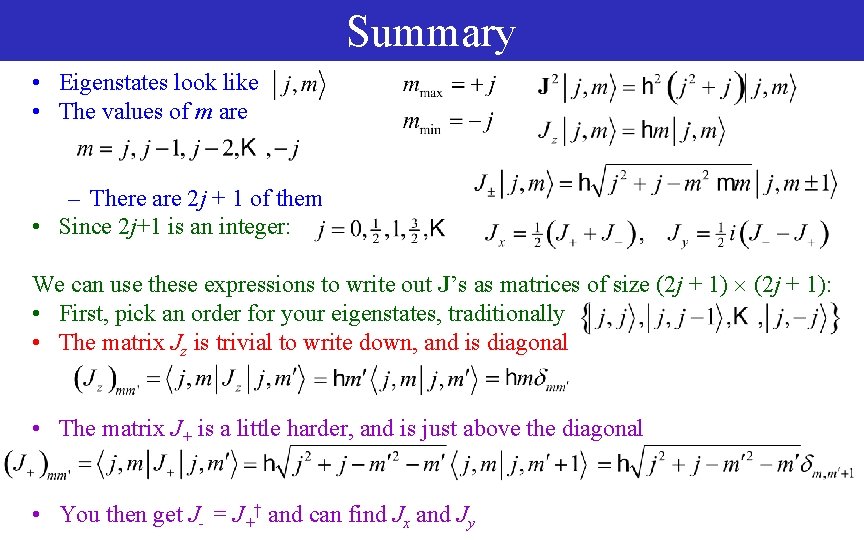
Summary • Eigenstates look like • The values of m are – There are 2 j + 1 of them • Since 2 j+1 is an integer: We can use these expressions to write out J’s as matrices of size (2 j + 1): • First, pick an order for your eigenstates, traditionally • The matrix Jz is trivial to write down, and is diagonal • The matrix J+ is a little harder, and is just above the diagonal • You then get J- = J+† and can find Jx and Jy

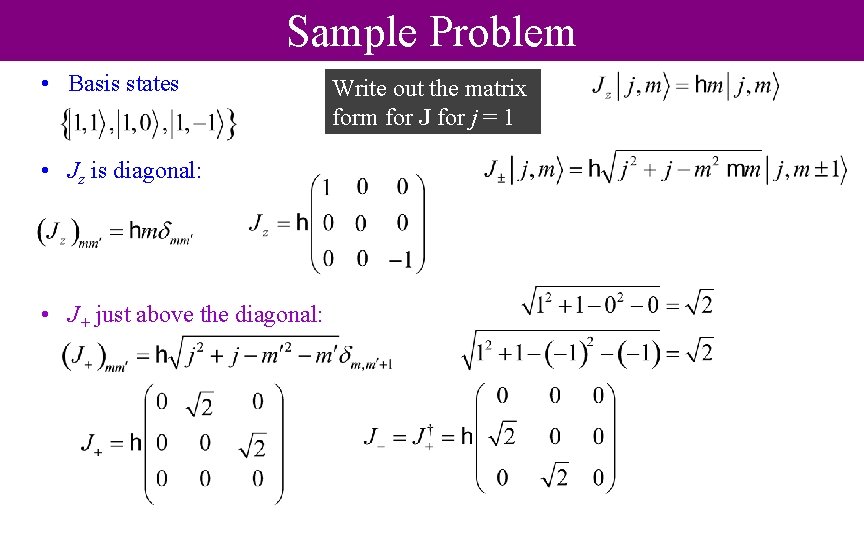
Sample Problem • Basis states • Jz is diagonal: • J+ just above the diagonal: Write out the matrix form for J for j = 1

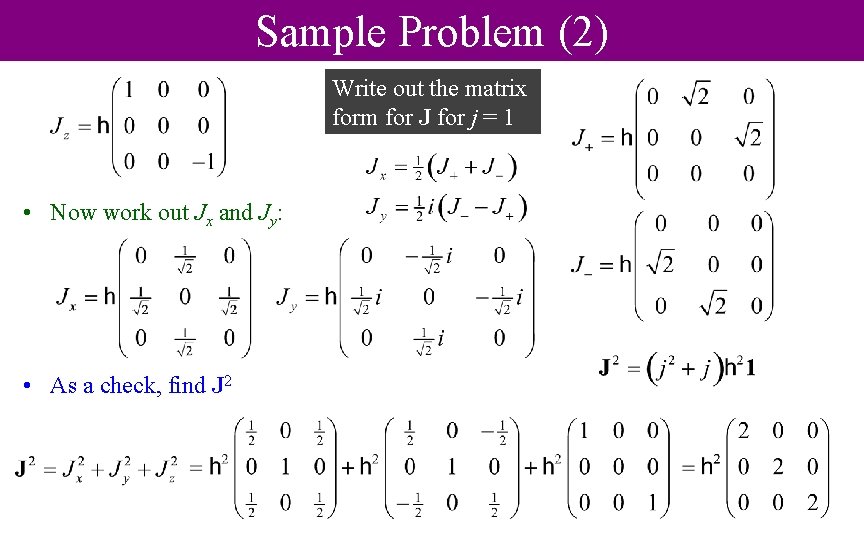
Sample Problem (2) Write out the matrix form for J for j = 1 • Now work out Jx and Jy: • As a check, find J 2

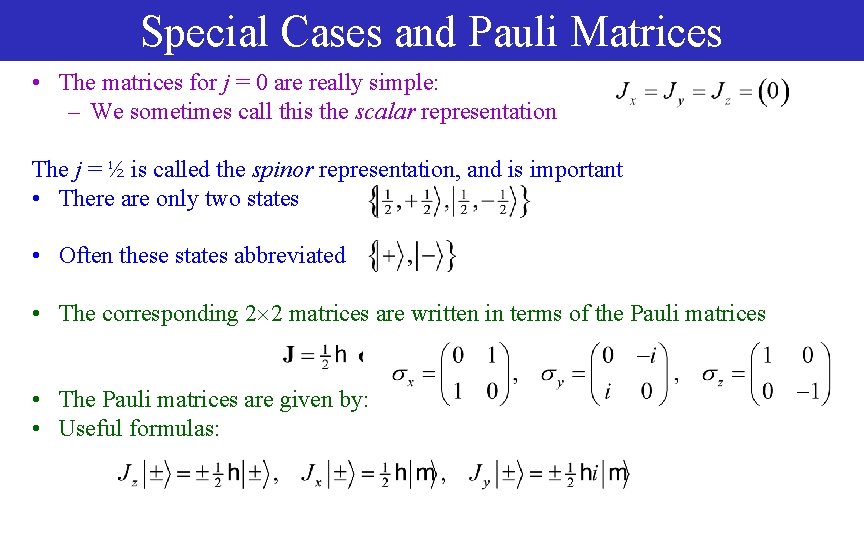
Special Cases and Pauli Matrices • The matrices for j = 0 are really simple: – We sometimes call this the scalar representation The j = ½ is called the spinor representation, and is important • There are only two states • Often these states abbreviated • The corresponding 2 2 matrices are written in terms of the Pauli matrices • The Pauli matrices are given by: • Useful formulas:

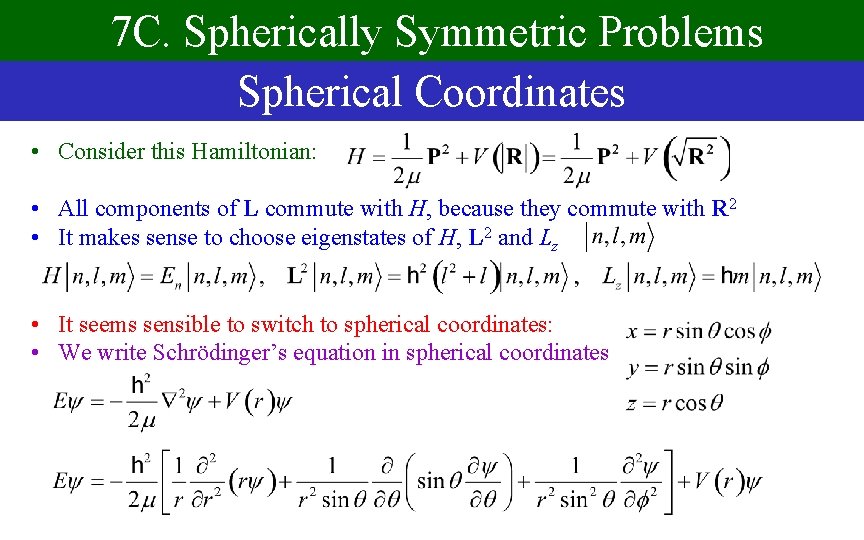
7 C. Spherically Symmetric Problems Spherical Coordinates • Consider this Hamiltonian: • All components of L commute with H, because they commute with R 2 • It makes sense to choose eigenstates of H, L 2 and Lz • It seems sensible to switch to spherical coordinates: • We write Schrödinger’s equation in spherical coordinates

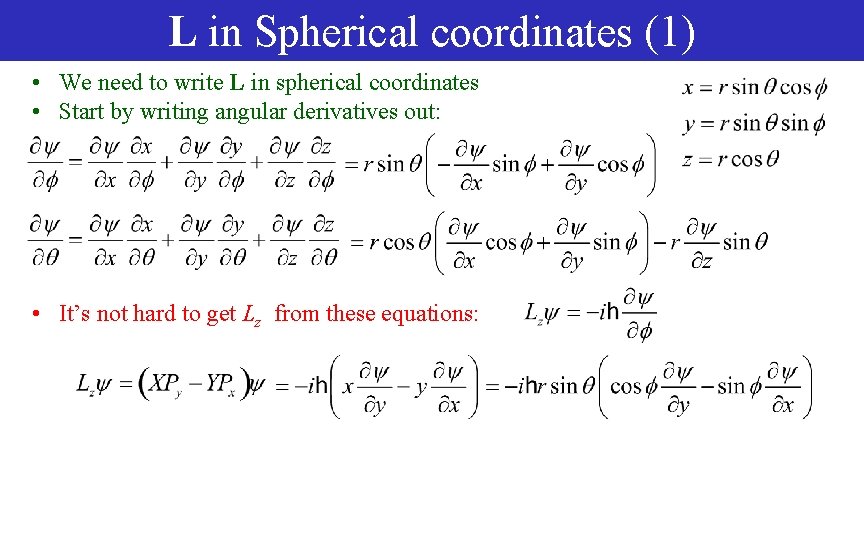
L in Spherical coordinates (1) • We need to write L in spherical coordinates • Start by writing angular derivatives out: • It’s not hard to get Lz from these equations:

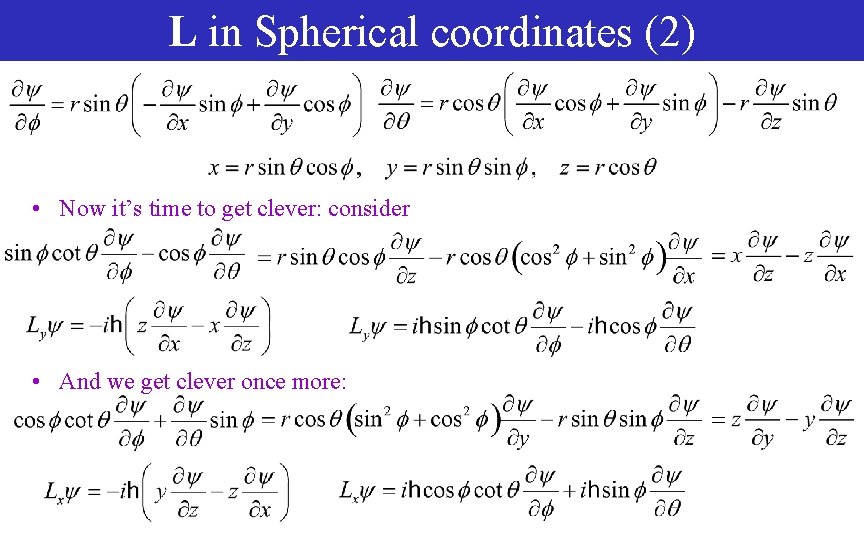
L in Spherical coordinates (2) • Now it’s time to get clever: consider • And we get clever once more:

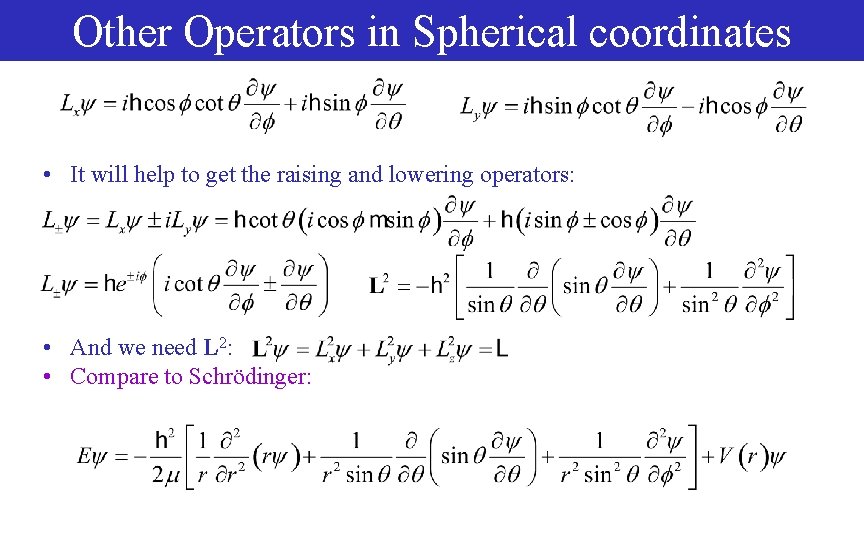
Other Operators in Spherical coordinates • It will help to get the raising and lowering operators: • And we need L 2: • Compare to Schrödinger:

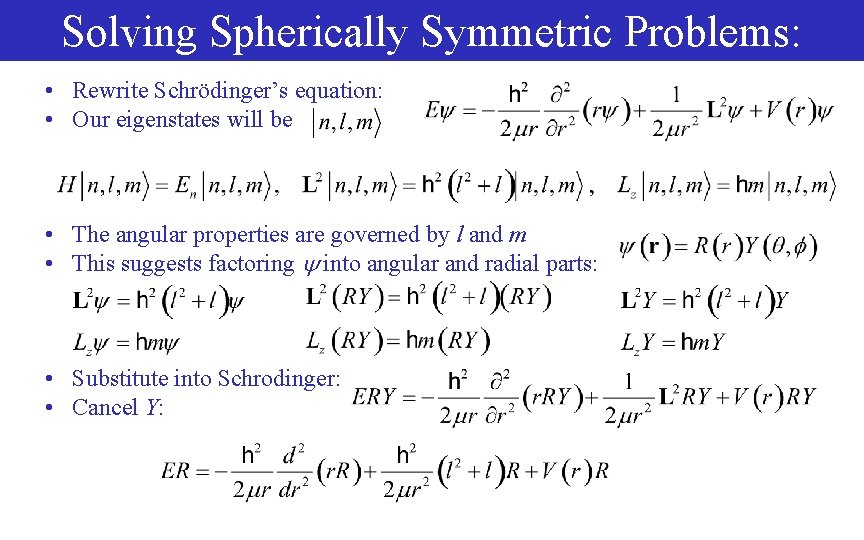
Solving Spherically Symmetric Problems: • Rewrite Schrödinger’s equation: • Our eigenstates will be • The angular properties are governed by l and m • This suggests factoring into angular and radial parts: • Substitute into Schrodinger: • Cancel Y:

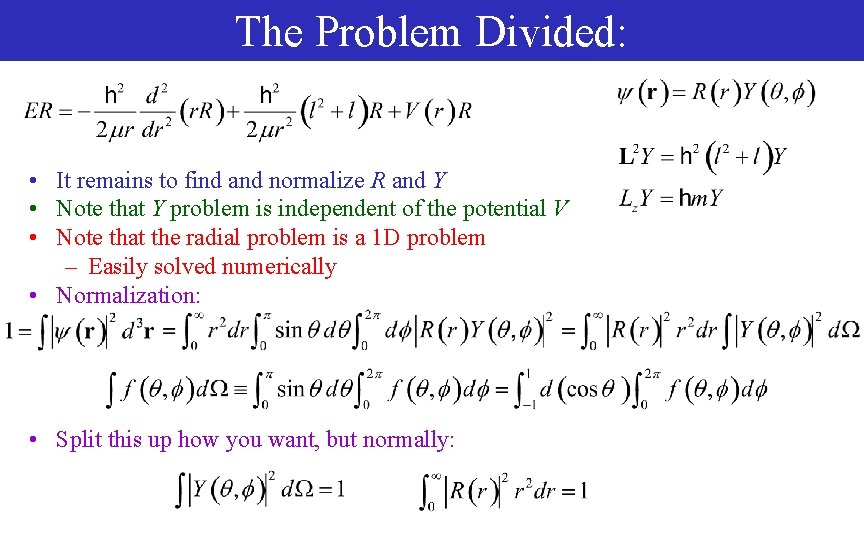
The Problem Divided: • It remains to find and normalize R and Y • Note that Y problem is independent of the potential V • Note that the radial problem is a 1 D problem – Easily solved numerically • Normalization: • Split this up how you want, but normally:

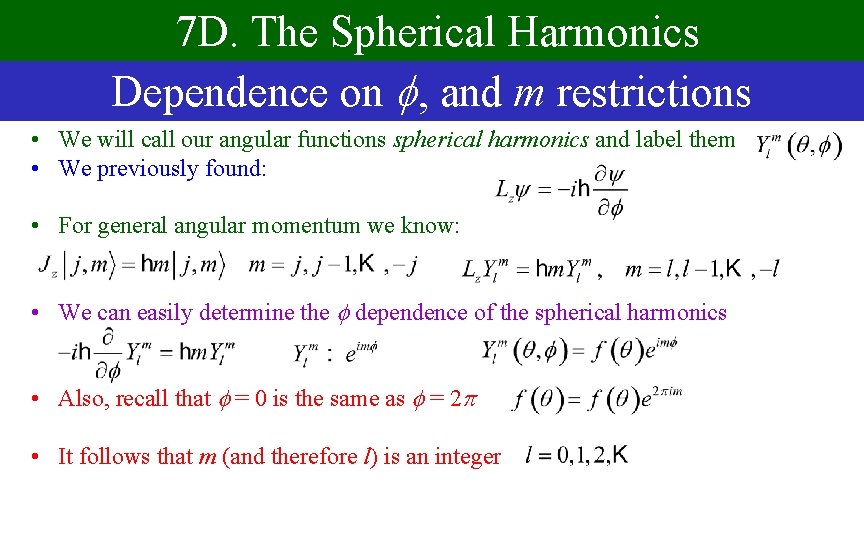
7 D. The Spherical Harmonics Dependence on , and m restrictions • We will call our angular functions spherical harmonics and label them • We previously found: • For general angular momentum we know: • We can easily determine the dependence of the spherical harmonics • Also, recall that = 0 is the same as = 2 • It follows that m (and therefore l) is an integer

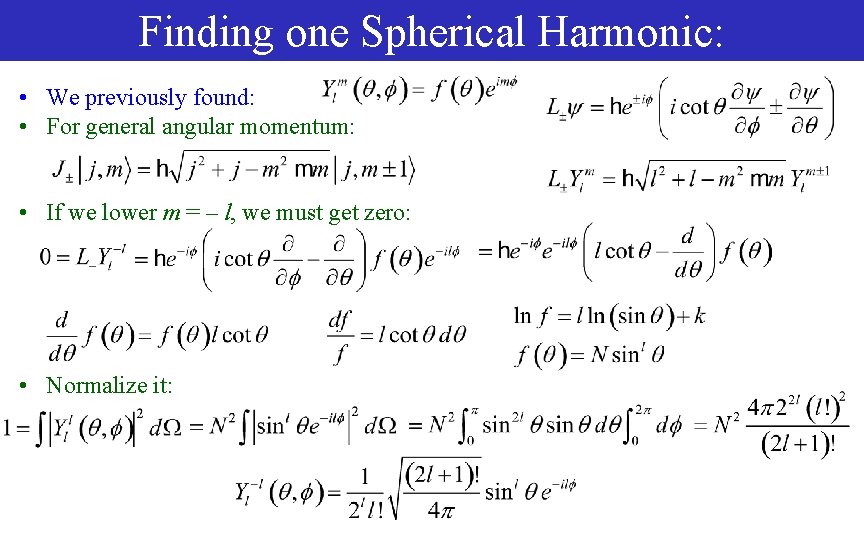
Finding one Spherical Harmonic: • We previously found: • For general angular momentum: • If we lower m = – l, we must get zero: • Normalize it:

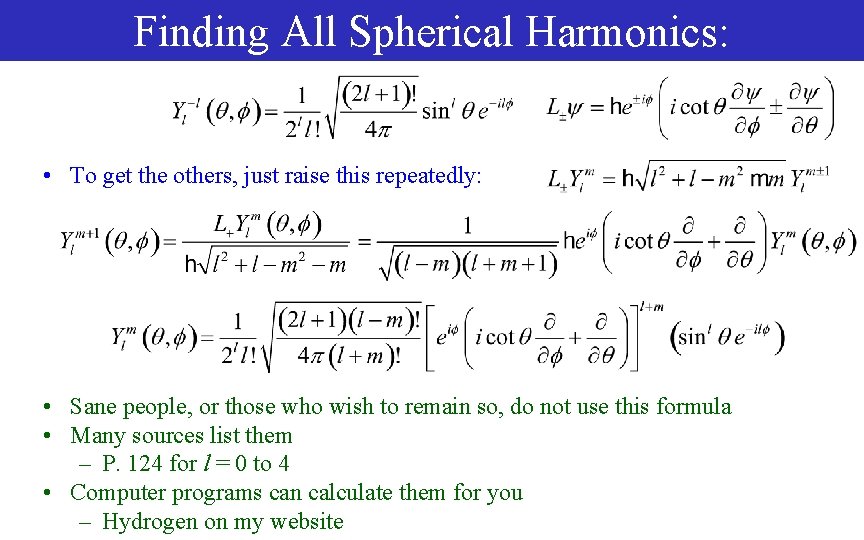
Finding All Spherical Harmonics: • To get the others, just raise this repeatedly: • Sane people, or those who wish to remain so, do not use this formula • Many sources list them – P. 124 for l = 0 to 4 • Computer programs can calculate them for you – Hydrogen on my website

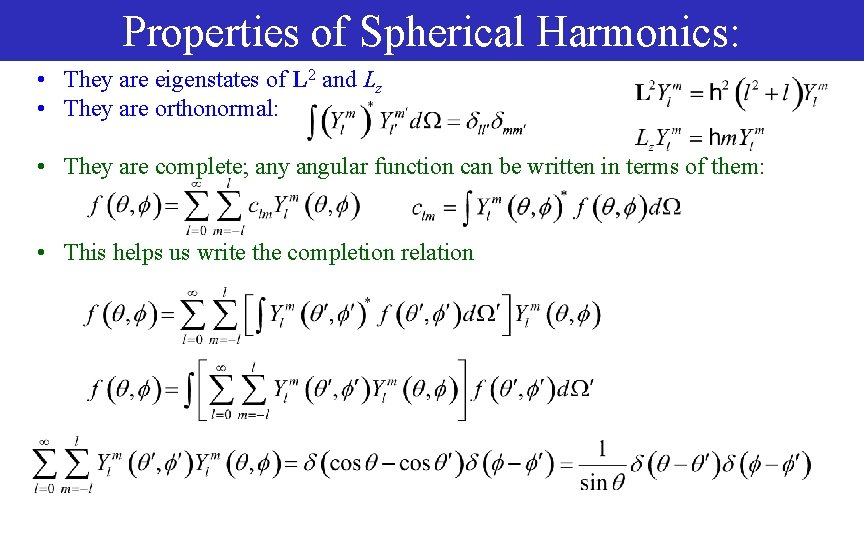
Properties of Spherical Harmonics: • They are eigenstates of L 2 and Lz • They are orthonormal: • They are complete; any angular function can be written in terms of them: • This helps us write the completion relation

More Properties of Spherical Harmonics: • Recall: parity commutes with L • It follows that • Hence when you let parity act, you must be getting essentially the same state • • Recall: L 2 is real but Lz is pure imaginary Take the complex conjugate of our relations above: This implies It works out to

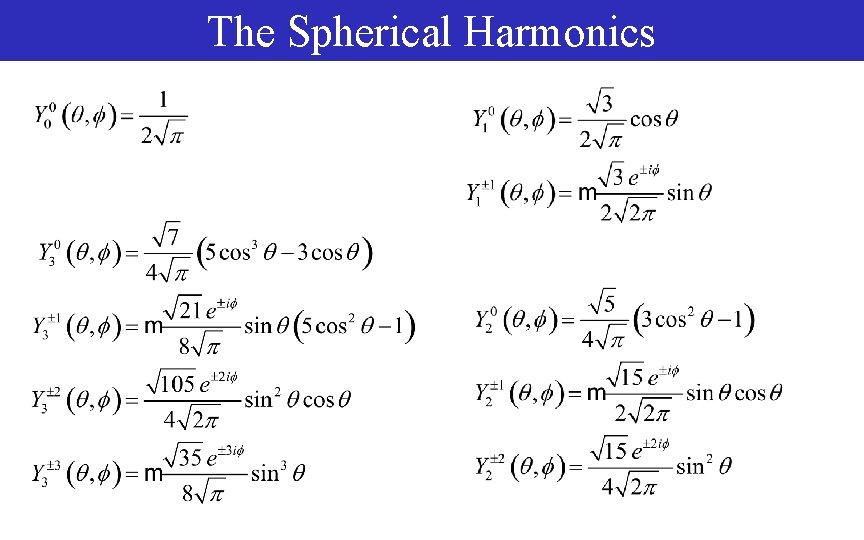
The Spherical Harmonics

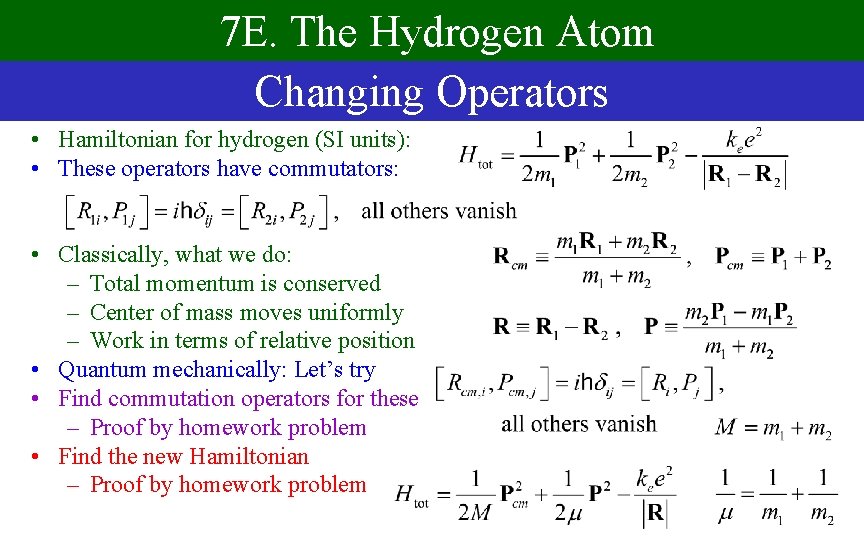
7 E. The Hydrogen Atom Changing Operators • Hamiltonian for hydrogen (SI units): • These operators have commutators: • Classically, what we do: – Total momentum is conserved – Center of mass moves uniformly – Work in terms of relative position • Quantum mechanically: Let’s try • Find commutation operators for these – Proof by homework problem • Find the new Hamiltonian – Proof by homework problem

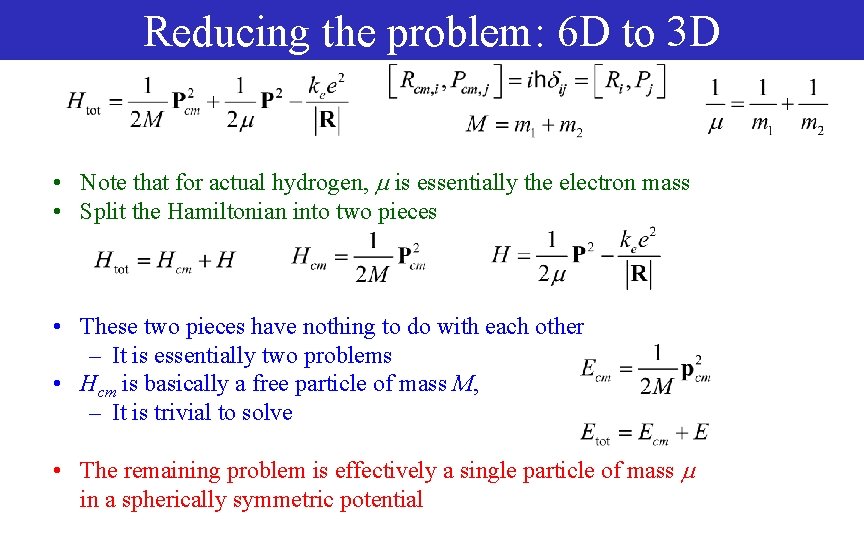
Reducing the problem: 6 D to 3 D • Note that for actual hydrogen, is essentially the electron mass • Split the Hamiltonian into two pieces • These two pieces have nothing to do with each other – It is essentially two problems • Hcm is basically a free particle of mass M, – It is trivial to solve • The remaining problem is effectively a single particle of mass in a spherically symmetric potential

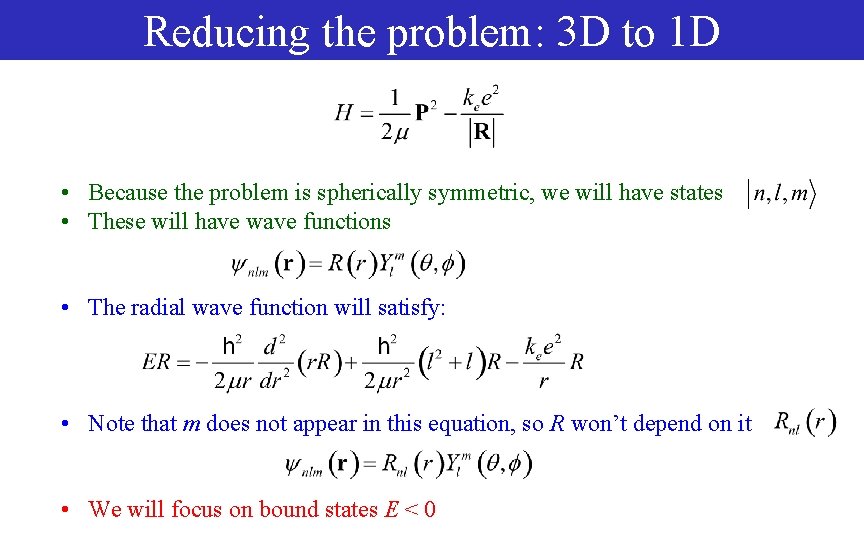
Reducing the problem: 3 D to 1 D • Because the problem is spherically symmetric, we will have states • These will have wave functions • The radial wave function will satisfy: • Note that m does not appear in this equation, so R won’t depend on it • We will focus on bound states E < 0

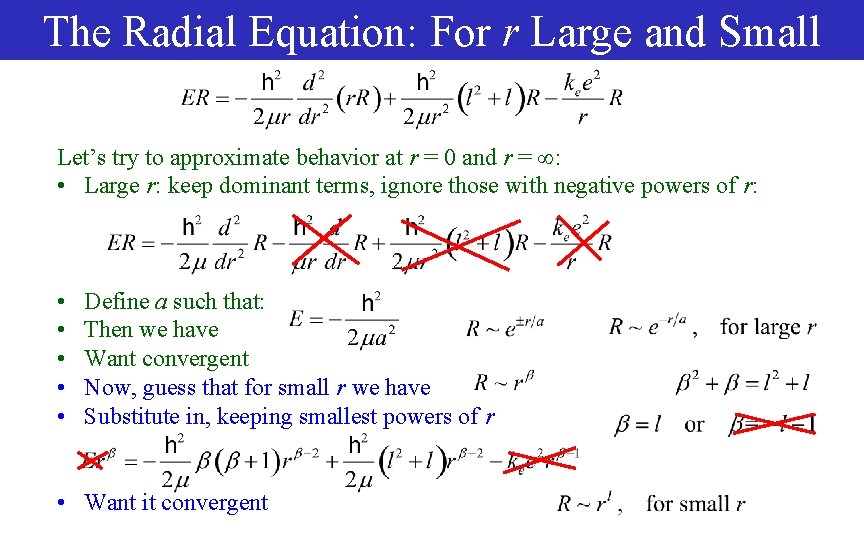
The Radial Equation: For r Large and Small Let’s try to approximate behavior at r = 0 and r = : • Large r: keep dominant terms, ignore those with negative powers of r: • • • Define a such that: Then we have Want convergent Now, guess that for small r we have Substitute in, keeping smallest powers of r • Want it convergent

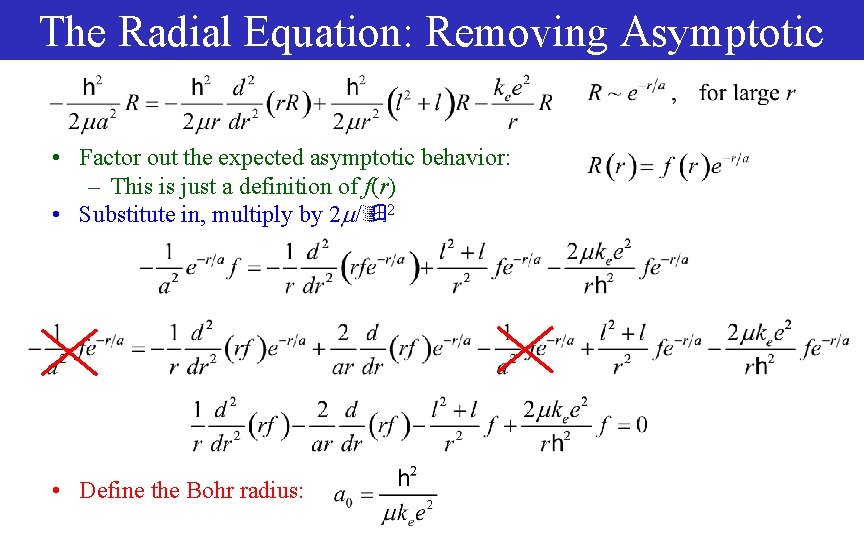
The Radial Equation: Removing Asymptotic • Factor out the expected asymptotic behavior: – This is just a definition of f(r) • Substitute in, multiply by 2 / 2 • Define the Bohr radius:

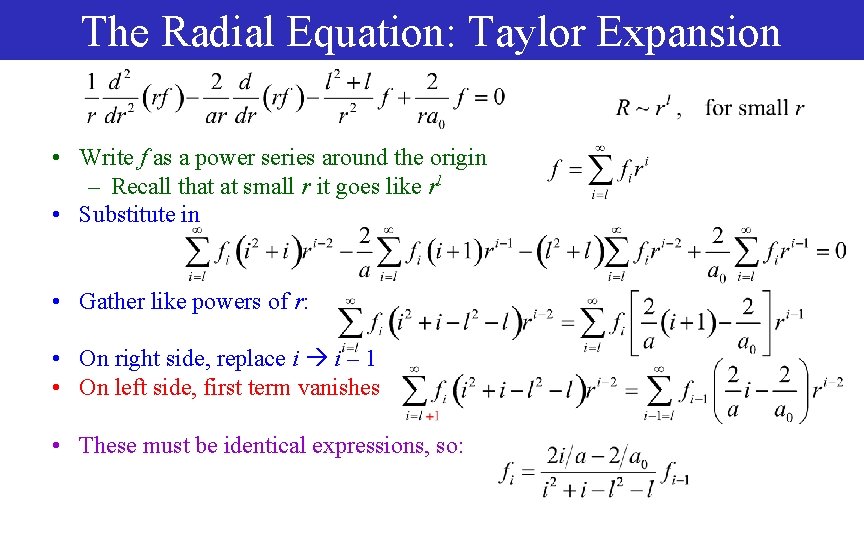
The Radial Equation: Taylor Expansion • Write f as a power series around the origin – Recall that at small r it goes like rl • Substitute in • Gather like powers of r: • On right side, replace i i – 1 • On left side, first term vanishes • These must be identical expressions, so:

Are We Done? • It looks like we have a solution for any E: – Pick fl to be anything – Deduce the rest by recursion • Now just normalize everything • Problem: No guarantee it is normalizable • Study the behavior at large i: • Only way to avoid this catastrophe is to make sure some f vanishes, say fn

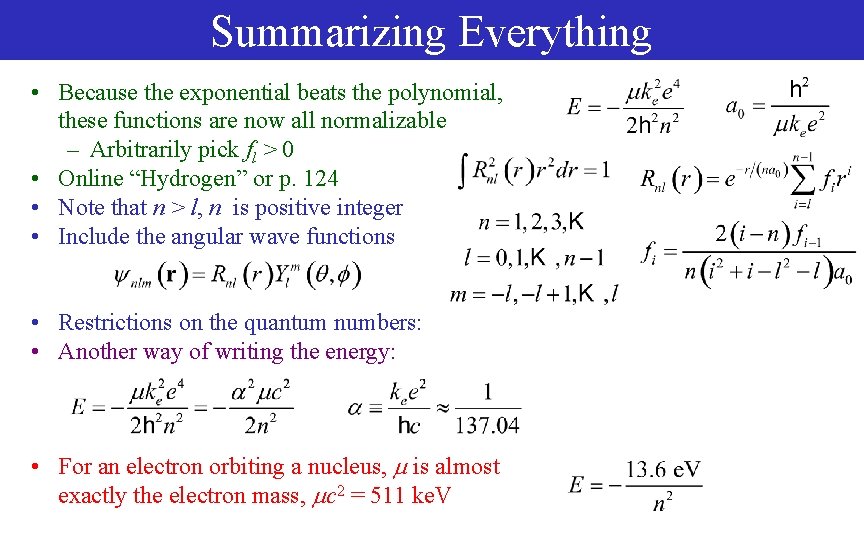
Summarizing Everything • Because the exponential beats the polynomial, these functions are now all normalizable – Arbitrarily pick fl > 0 • Online “Hydrogen” or p. 124 • Note that n > l, n is positive integer • Include the angular wave functions • Restrictions on the quantum numbers: • Another way of writing the energy: • For an electron orbiting a nucleus, is almost exactly the electron mass, c 2 = 511 ke. V

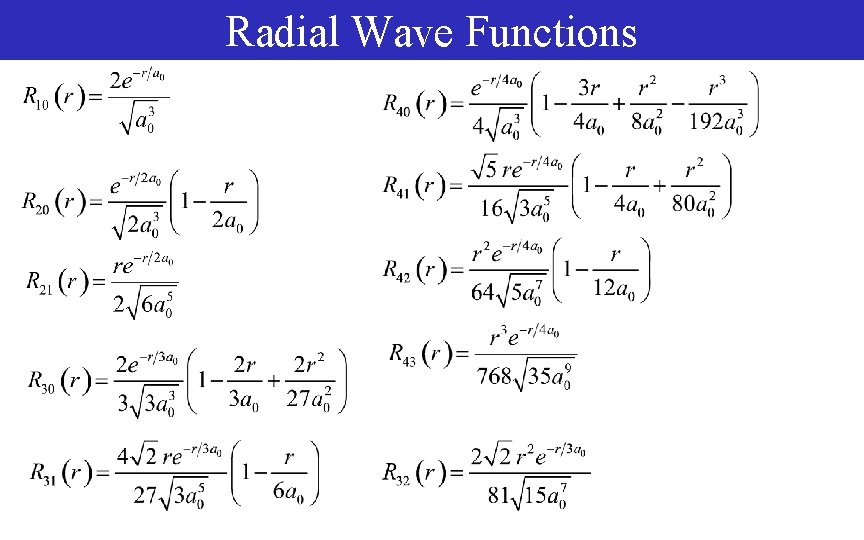
Radial Wave Functions

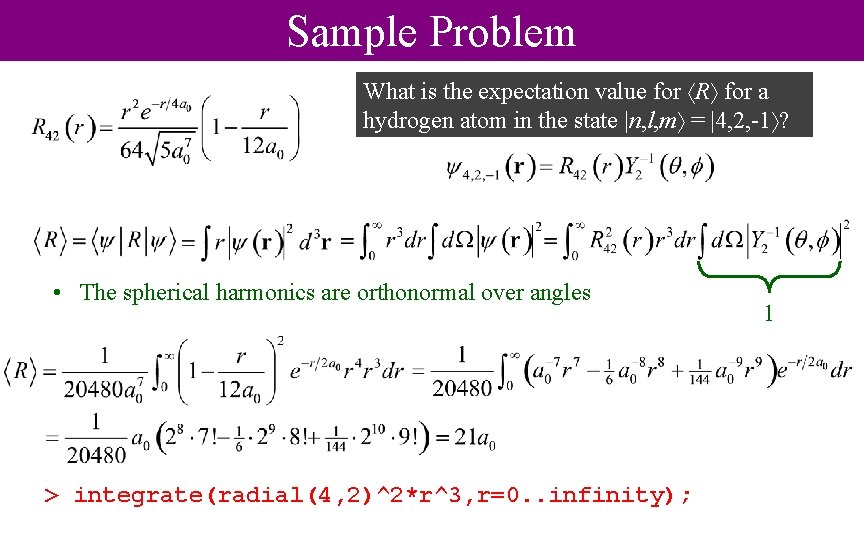
Sample Problem What is the expectation value for R for a hydrogen atom in the state |n, l, m = |4, 2, -1 ? • The spherical harmonics are orthonormal over angles > integrate(radial(4, 2)^2*r^3, r=0. . infinity); 1

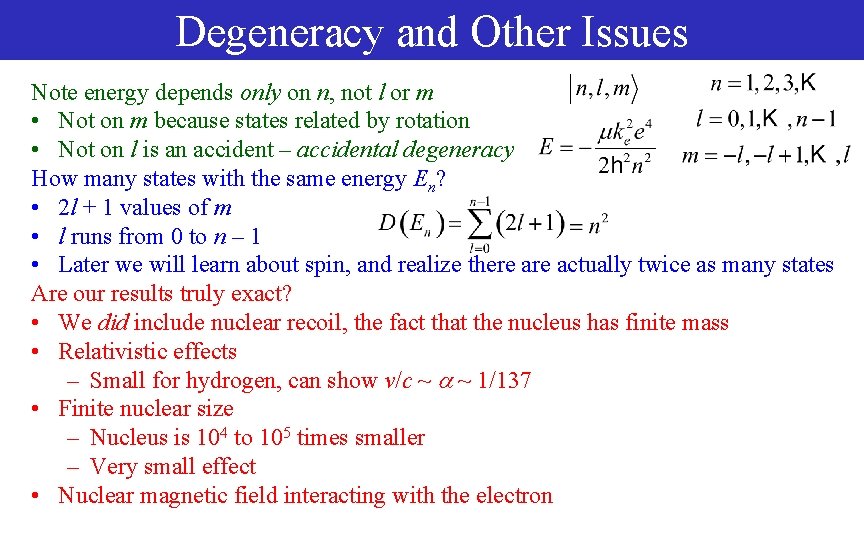
Degeneracy and Other Issues Note energy depends only on n, not l or m • Not on m because states related by rotation • Not on l is an accident – accidental degeneracy How many states with the same energy En? • 2 l + 1 values of m • l runs from 0 to n – 1 • Later we will learn about spin, and realize there actually twice as many states Are our results truly exact? • We did include nuclear recoil, the fact that the nucleus has finite mass • Relativistic effects – Small for hydrogen, can show v/c ~ ~ 1/137 • Finite nuclear size – Nucleus is 104 to 105 times smaller – Very small effect • Nuclear magnetic field interacting with the electron

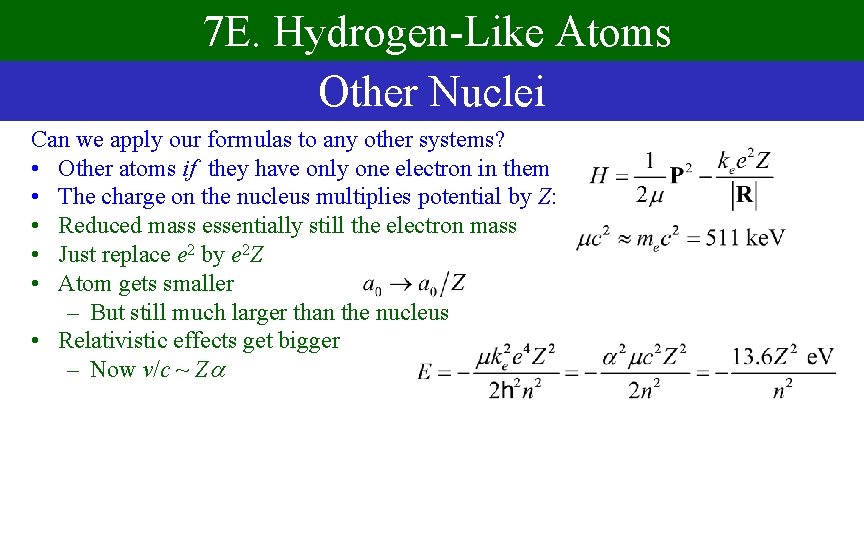
7 E. Hydrogen-Like Atoms Other Nuclei Can we apply our formulas to any other systems? • Other atoms if they have only one electron in them • The charge on the nucleus multiplies potential by Z: • Reduced mass essentially still the electron mass • Just replace e 2 by e 2 Z • Atom gets smaller – But still much larger than the nucleus • Relativistic effects get bigger – Now v/c ~ Z

Bizarre “atoms” We can replace the nucleus or the electron with other things Anti-muon plus electron • Anti-muon has same charge as proton, and much more mass than electron • Essentially identical with hydrogen Positronium = anti-electron (positron) plus electron • Same charge as proton • Positron’s mass = electron’s mass • Reduced mass and energy states reduced by half Nucleus plus muon • Muon 207 times heavier than electron • Atom is 207 times smaller • Even inside a complex atom, muon sees essentially bare nucleus • Atom small enough that for large Z, muon is partly inside nucleus Anti-hydrogen = anti-proton plus anti-electron • Identical to hydrogen