7 7 Standard Molar Entropies Standard Molar Entropy





- Slides: 10

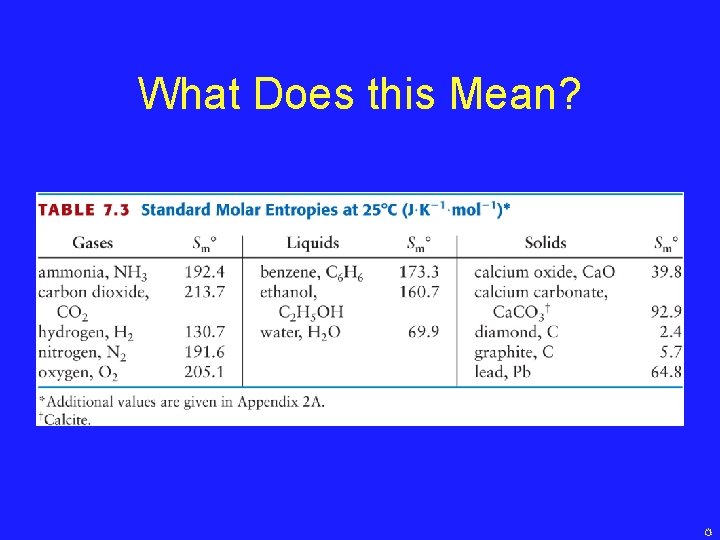
7. 7: Standard Molar Entropies • Standard Molar Entropy = Entropy of 1 mole of material at 298. 15 K and 1 bar • When we consider the standard molar entropies of molecules, we apply the following rule: Standard Molar Entropies Increase as the Complexity of a Substance Increases and The Standard Molar Entropies of gases are higher than those of liquids or solids at the same temperature

What Does this Mean?

7. 8: Standard Reaction Entropies • Entropy and the tendency for spontaneous processes to increase the entropy of a system can be used to predict if/how reactions will occur • We know from Sºm that one set of molecules will have lower entropy than another – Taking the same logic a step further will allow us to predict the change in entropy of a reaction

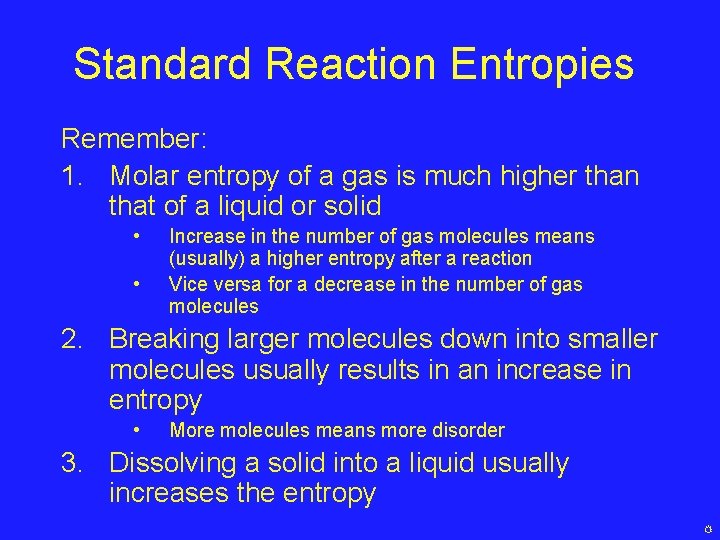
Standard Reaction Entropies Remember: 1. Molar entropy of a gas is much higher than that of a liquid or solid • • Increase in the number of gas molecules means (usually) a higher entropy after a reaction Vice versa for a decrease in the number of gas molecules 2. Breaking larger molecules down into smaller molecules usually results in an increase in entropy • More molecules means more disorder 3. Dissolving a solid into a liquid usually increases the entropy

Calculating the Entropy of a Reaction • Sometimes we can’t always use our judgment and we need to calculate the entropy • In order to do this, we need the standard molar entropies of the products and the reactants as well as the number of moles of each

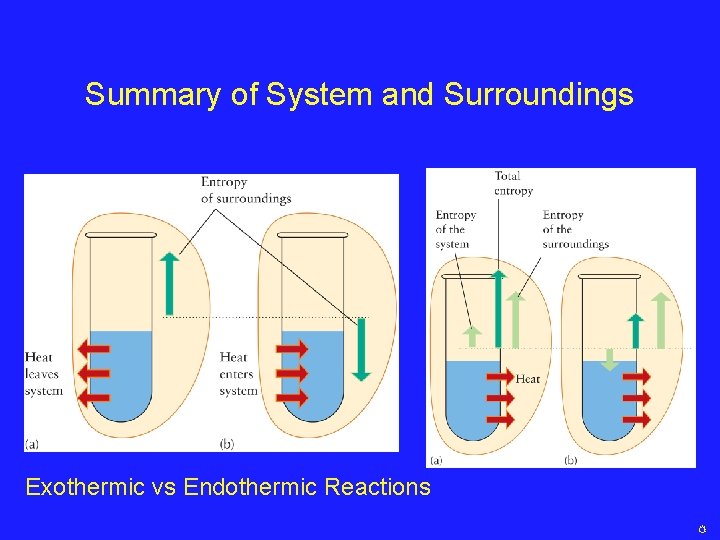
Global Changes in Entropy • We know about the role of entropy in chemical reactions and spontaneous processes • How can we use entropy to explain processes that spontaneously happen, but appear to go against the 2 nd Law? – Water freezing to ice – Cold packs becoming cold in the summer – Cells forming from the primordial seas • The contradiction is just a matter of scale…

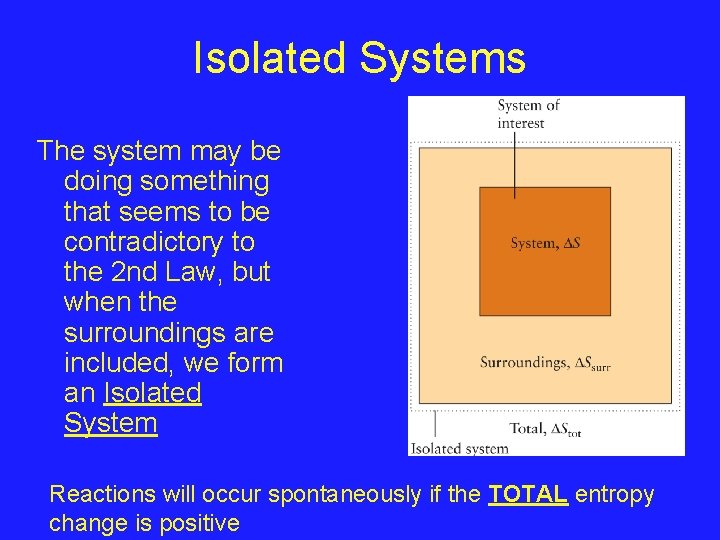
Isolated Systems The system may be doing something that seems to be contradictory to the 2 nd Law, but when the surroundings are included, we form an Isolated System Reactions will occur spontaneously if the TOTAL entropy change is positive

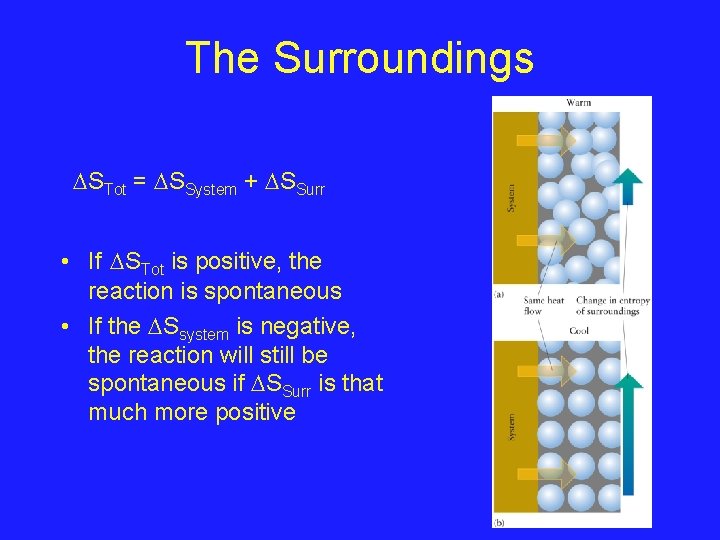
The Surroundings STot = SSystem + SSurr • If STot is positive, the reaction is spontaneous • If the Ssystem is negative, the reaction will still be spontaneous if SSurr is that much more positive

S and Enthalpy • We can better understand the role of the surroundings by looking at the boiling of water…

Summary of System and Surroundings Exothermic vs Endothermic Reactions