7 7 NOTES Shapes for Covalent Structures IV












- Slides: 14

7. 7 – NOTES Shapes for Covalent Structures

• IV. Molecular shape • A. VSEPR Model – Valence Shell Electron Pair Repulsion • e- pairs get as far apart as possible because of similar charges •

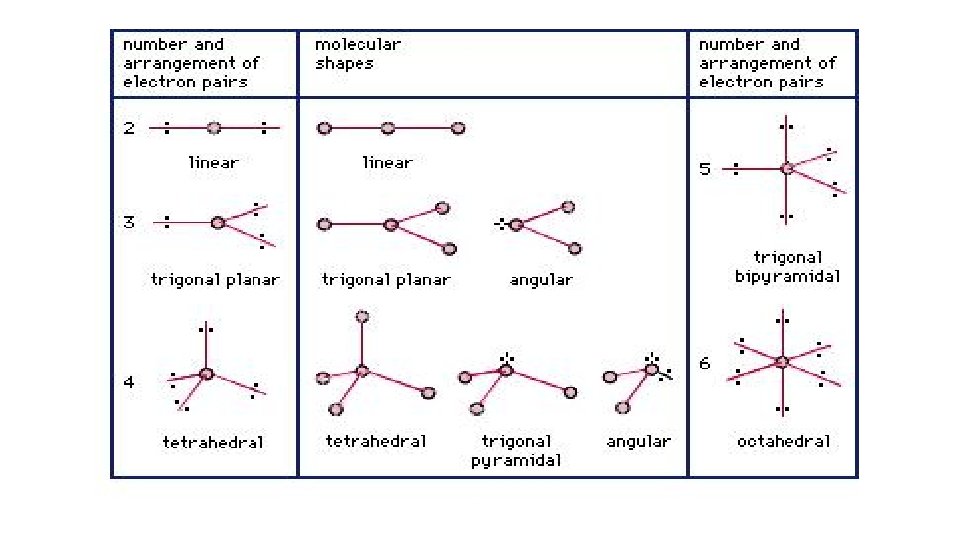
• B. To determine shapes of molecules: • 1. Draw the Lewis diagram for the compound. • 2. Count the number of electron clouds around the central atom. • Multiple bonds and single bonds are treated alike. Nonbonded e- pairs (lone pairs) are counted, too. • 3. Shapes are determined by the number of e- clouds:

• 2 clouds: CO 2 • 2 shared pairs (bonds), 0 unshared • Linear; 180° largest distance possible;

• 3 clouds: BH 3, SO 3 • 3 shared pairs, 0 unshared • 120° angle b/t electron clouds; called equilateral triangle or trigonal planar;

• 4 clouds: CH 4, Si. F 4 • 4 shared pairs, 0 unshared • 109. 5° angle, tetrahedron, angles do NOT have to = 360 unless flat circle

• *5 clouds: PF 5 • 5 shared, 0 unshared • 90° angles and 120° angles; trigonal bipyramid

• *6 clouds: SF 6 • 6 shared, 0 unshared • All angles are 90°; octahedron


• 4. “Shape” or geometry refers to the positions of the atoms only. If one or more electron clouds are non bonded, you must look at the atom positions alone. • Examples: H 2 O, NH 3, SO 2 • electron clouds determine angle; atoms determine shape; once know the shape, draw it as such; • Unshared pairs repel bonding pairs! • **Angular = bent

• C. Hybridization • In order to make bond angles larger (less repulsion), atoms blend their orbitals into hybrids.

• Determining the hybridization: • 1. The orbitals available for bonding are s, p, and d. Listing them one orbital at a time: s, p, p, p, d, d, d. • 2. For a particular compound, draw the Lewis structure, then count the number of e- cloud around an atom, including lone pairs. Count double bonds as 1 cloud. The atom needs 1 orbital for each cloud. • 3. For 2 clouds, 2 orbitals are needed, so the hybrids are sp. • For 3 clouds, 3 orbitals are needed, so the hybrids are sp 2. • For 4 clouds, the hybridization is sp 3; for 5 sp 3 d, and for 6 sp 3 d 2. •


• CO 2 • CH 4 • NH 3