7 5 The First Law of Thermodynamics Internal
- Slides: 17

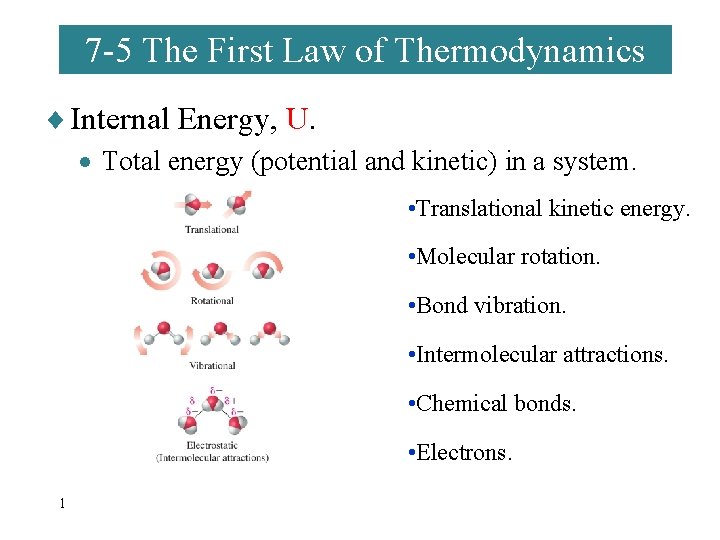
7 -5 The First Law of Thermodynamics ¨ Internal Energy, U. · Total energy (potential and kinetic) in a system. • Translational kinetic energy. • Molecular rotation. • Bond vibration. • Intermolecular attractions. • Chemical bonds. • Electrons. 1

First Law of Thermodynamics ¨ A system contains only internal energy. · A system does not contain heat or work. · These only occur during a change in the system. U = q + w ¨ Law of Conservation of Energy · The energy of an isolated system is constant 2

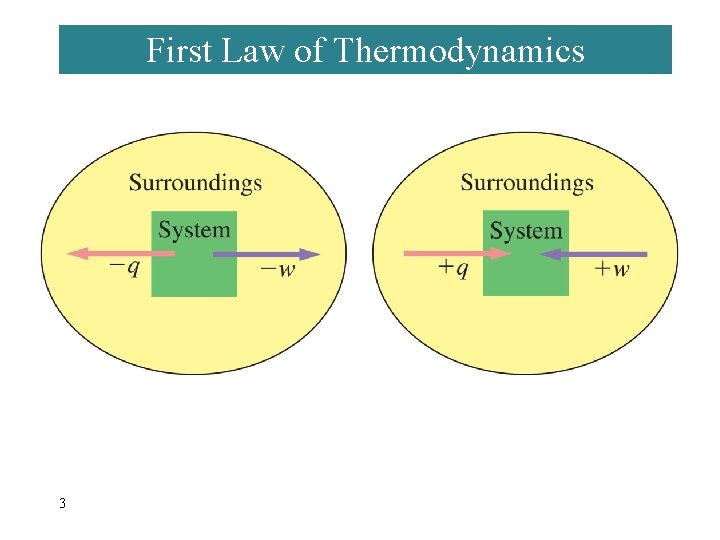
First Law of Thermodynamics 3

Functions of State ¨ Any property that has a unique value for a specified state of a system is said to be a State Function. ◦ ◦ 4 Water at 293. 15 K and 1. 00 atm is in a specified state. d = 0. 99820 g/m. L This density is a unique function of the state. It does not matter how the state was established.

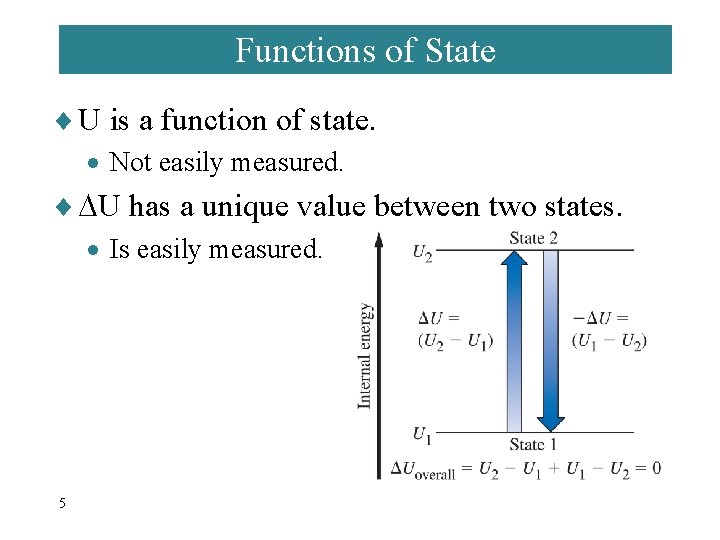
Functions of State ¨ U is a function of state. · Not easily measured. ¨ U has a unique value between two states. · Is easily measured. 5

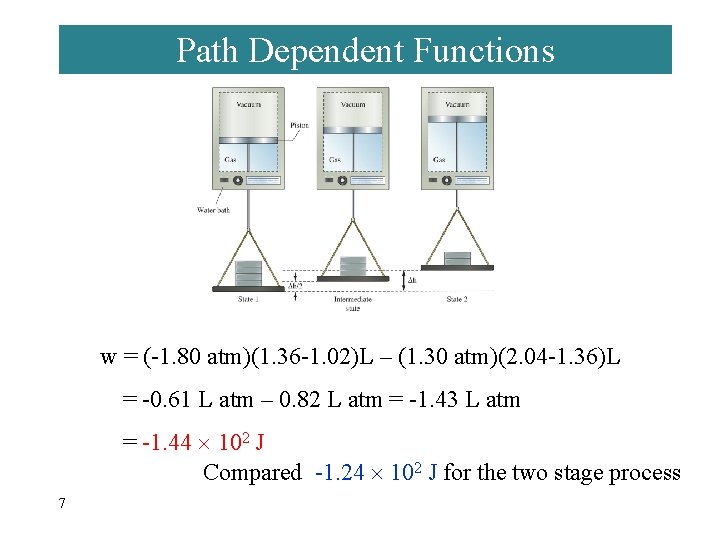
Path Dependent Functions ¨ Changes in heat and work are not functions of state. · Remember example 7 -5, w = -1. 24 102 J in a one step expansion of gas: · Consider 2. 40 atm to 1. 80 atm and finally to 1. 20 atm. 6

Path Dependent Functions w = (-1. 80 atm)(1. 36 -1. 02)L – (1. 30 atm)(2. 04 -1. 36)L = -0. 61 L atm – 0. 82 L atm = -1. 43 L atm = -1. 44 102 J Compared -1. 24 102 J for the two stage process 7

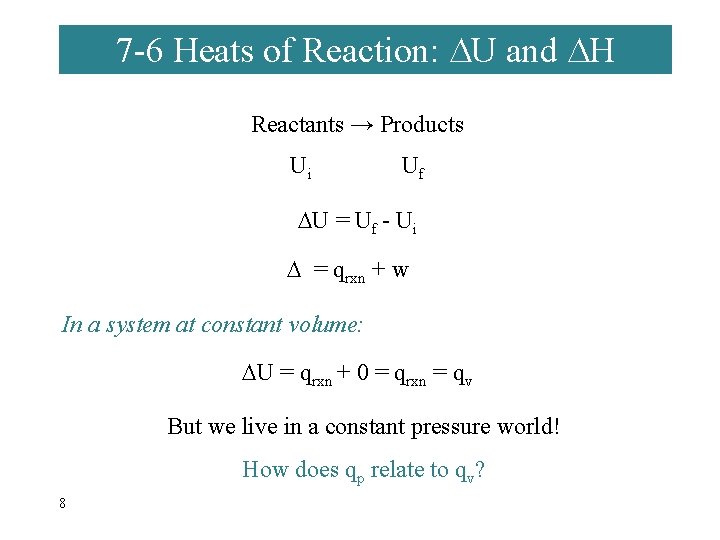
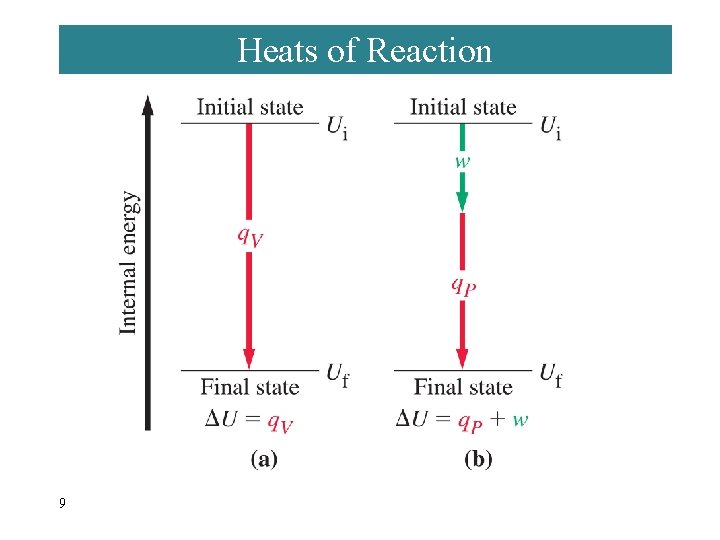
7 -6 Heats of Reaction: U and H Reactants → Products Ui Uf U = Uf - Ui = qrxn + w In a system at constant volume: U = qrxn + 0 = qrxn = qv But we live in a constant pressure world! How does qp relate to qv? 8

Heats of Reaction 9

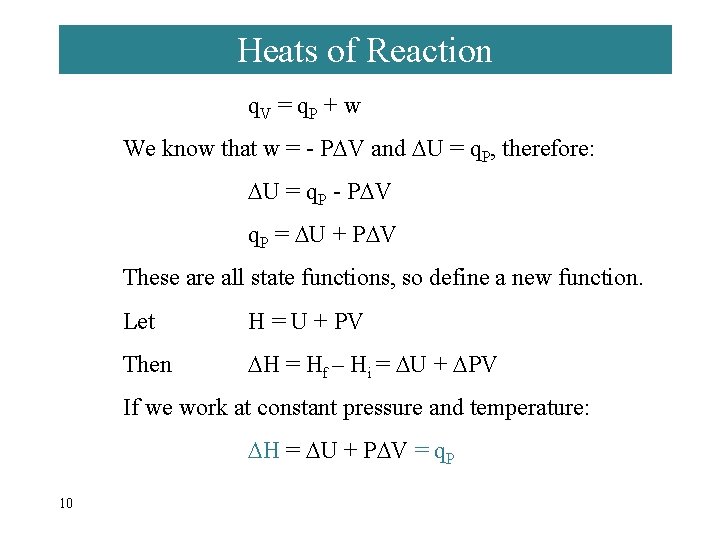
Heats of Reaction q. V = q. P + w We know that w = - P V and U = q. P, therefore: U = q. P - P V q. P = U + P V These are all state functions, so define a new function. Let H = U + PV Then H = Hf – Hi = U + PV If we work at constant pressure and temperature: H = U + P V = q. P 10

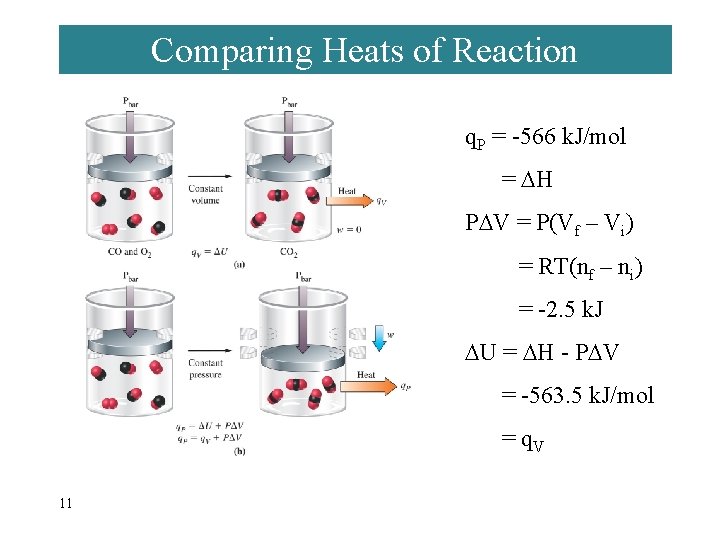
Comparing Heats of Reaction q. P = -566 k. J/mol = H P V = P(Vf – Vi) = RT(nf – ni) = -2. 5 k. J U = H - P V = -563. 5 k. J/mol = q. V 11

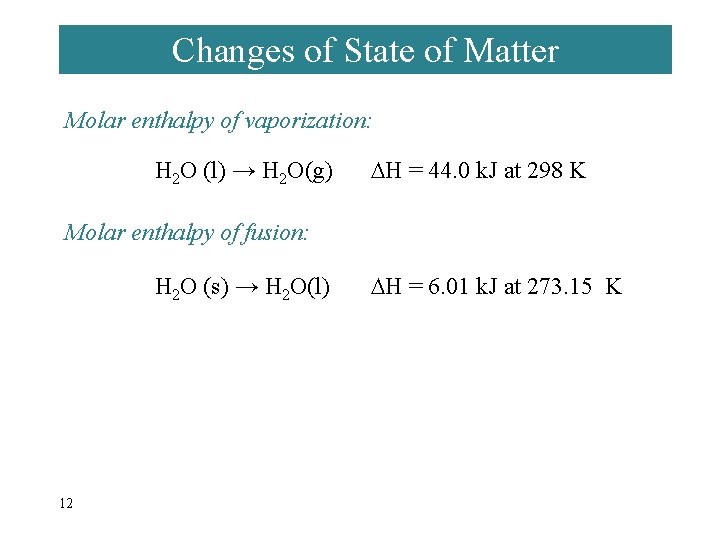
Changes of State of Matter Molar enthalpy of vaporization: H 2 O (l) → H 2 O(g) H = 44. 0 k. J at 298 K Molar enthalpy of fusion: H 2 O (s) → H 2 O(l) 12 H = 6. 01 k. J at 273. 15 K

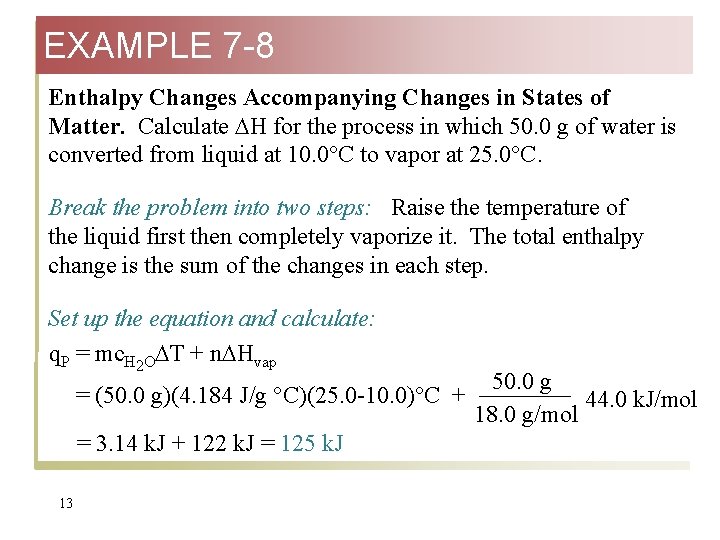
EXAMPLE 7 -8 Enthalpy Changes Accompanying Changes in States of Matter. Calculate H for the process in which 50. 0 g of water is converted from liquid at 10. 0°C to vapor at 25. 0°C. Break the problem into two steps: Raise the temperature of the liquid first then completely vaporize it. The total enthalpy change is the sum of the changes in each step. Set up the equation and calculate: q. P = mc. H 2 O T + n Hvap = (50. 0 g)(4. 184 J/g °C)(25. 0 -10. 0)°C + = 3. 14 k. J + 122 k. J = 125 k. J 13 50. 0 g 44. 0 k. J/mol 18. 0 g/mol

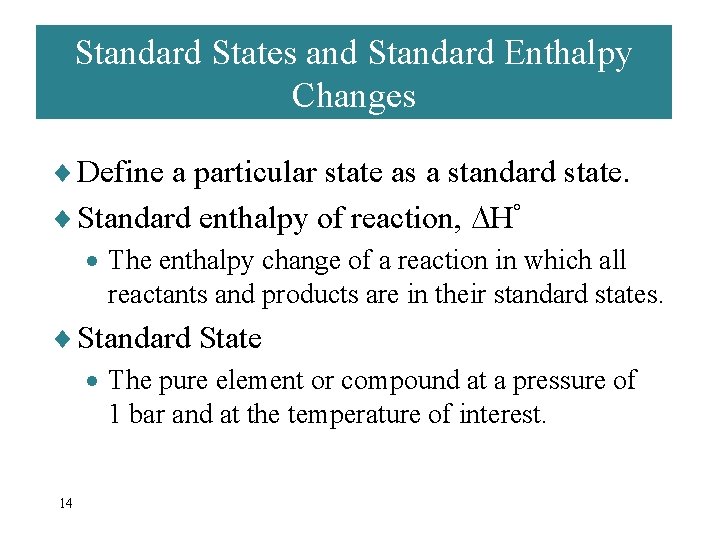
Standard States and Standard Enthalpy Changes ¨ Define a particular state as a standard state. ¨ Standard enthalpy of reaction, H° · The enthalpy change of a reaction in which all reactants and products are in their standard states. ¨ Standard State · The pure element or compound at a pressure of 1 bar and at the temperature of interest. 14

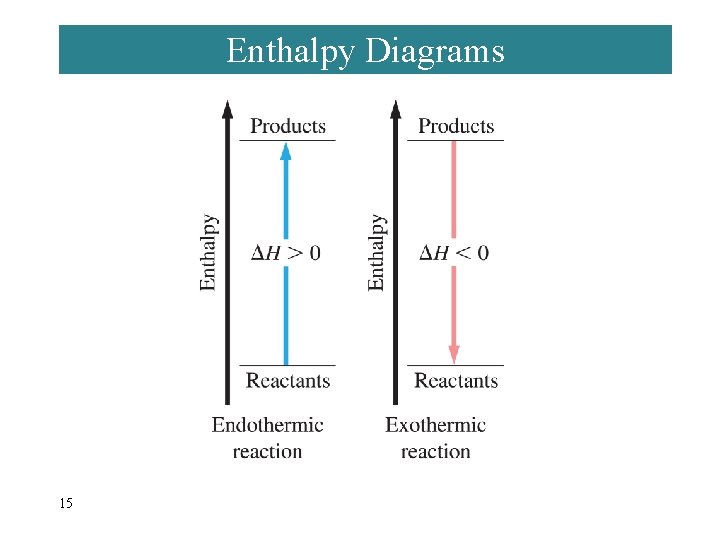
Enthalpy Diagrams 15

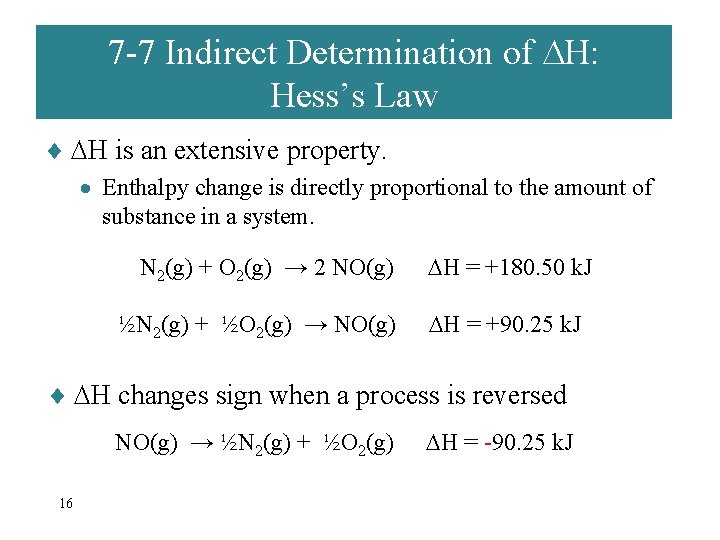
7 -7 Indirect Determination of H: Hess’s Law ¨ H is an extensive property. · Enthalpy change is directly proportional to the amount of substance in a system. N 2(g) + O 2(g) → 2 NO(g) ½N 2(g) + ½O 2(g) → NO(g) H = +180. 50 k. J H = +90. 25 k. J ¨ H changes sign when a process is reversed NO(g) → ½N 2(g) + ½O 2(g) 16 H = -90. 25 k. J

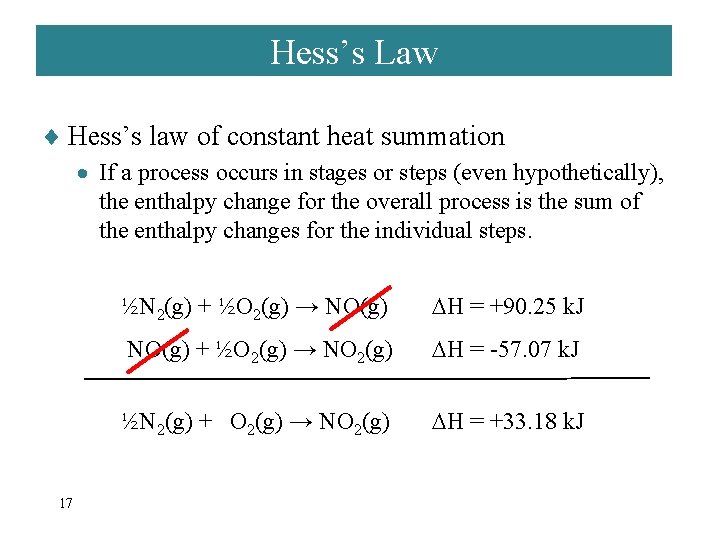
Hess’s Law ¨ Hess’s law of constant heat summation · If a process occurs in stages or steps (even hypothetically), the enthalpy change for the overall process is the sum of the enthalpy changes for the individual steps. 17 ½N 2(g) + ½O 2(g) → NO(g) H = +90. 25 k. J NO(g) + ½O 2(g) → NO 2(g) H = -57. 07 k. J ½N 2(g) + O 2(g) → NO 2(g) H = +33. 18 k. J