7 4 NOTES Metallic Bonding and Emperical Formulas

7. 4 – NOTES Metallic Bonding and Emperical Formulas

• V. Empirical Formula • A. Empirical formula • Definition – simplest, whole number ratio of atoms If you know the percentage composition of a compound, then you will be able to determine the empirical formula (simplest ratio of elements) by using mole relationships.

• Steps in the process: • 1. Assume a 100 gram sample. Then each percentage is equal to grams. (e. g. 41% would become 41 g) • 2. Change the grams to moles. • 3. Determine the empirical formula by dividing all the moles by the smallest number of moles. If the empirical formula contains nonwhole numbers, multiply all moles by a factor that makes all whole numbers.

• Examples: • 1. A compound of sulfur and oxygen is 50. 0% sulfur and 50. 0% oxygen by mass. What is the empirical formula? • S – 50. 0 g (1 mol/ 32. 1 g) = 1. 55763 / 1. 55763 = 1 • O – 50. 0 g (1 mol/ 16. 0 g) = 3. 125 / 1. 55763 = 2 • SO 2
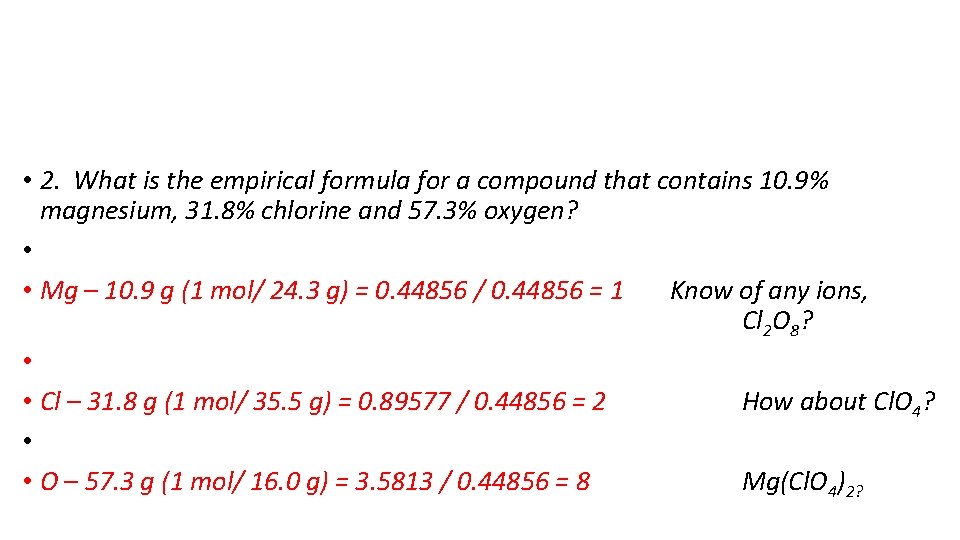
• 2. What is the empirical formula for a compound that contains 10. 9% magnesium, 31. 8% chlorine and 57. 3% oxygen? • • Mg – 10. 9 g (1 mol/ 24. 3 g) = 0. 44856 / 0. 44856 = 1 Know of any ions, Cl 2 O 8? • • Cl – 31. 8 g (1 mol/ 35. 5 g) = 0. 89577 / 0. 44856 = 2 How about Cl. O 4? • • O – 57. 3 g (1 mol/ 16. 0 g) = 3. 5813 / 0. 44856 = 8 Mg(Cl. O 4)2?
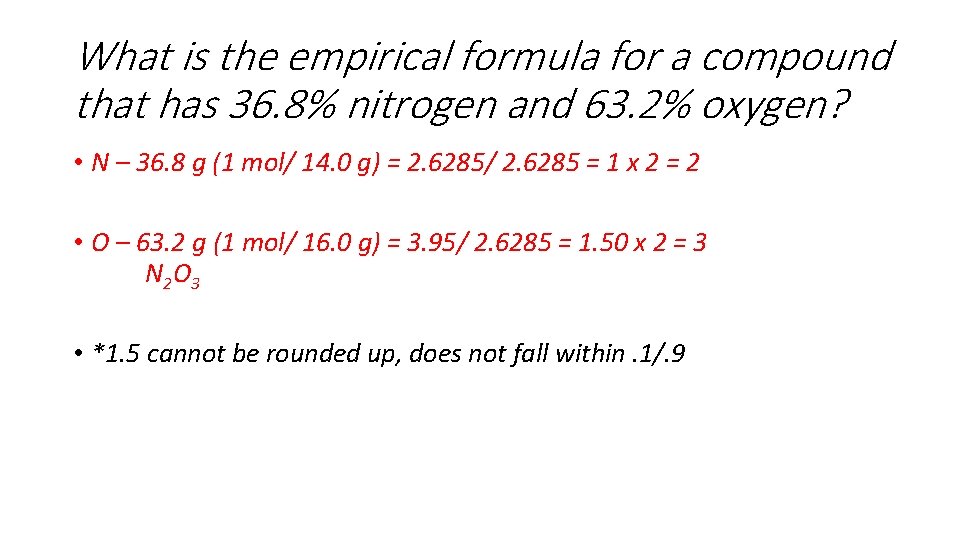
What is the empirical formula for a compound that has 36. 8% nitrogen and 63. 2% oxygen? • N – 36. 8 g (1 mol/ 14. 0 g) = 2. 6285/ 2. 6285 = 1 x 2 = 2 • O – 63. 2 g (1 mol/ 16. 0 g) = 3. 95/ 2. 6285 = 1. 50 x 2 = 3 N 2 O 3 • *1. 5 cannot be rounded up, does not fall within. 1/. 9
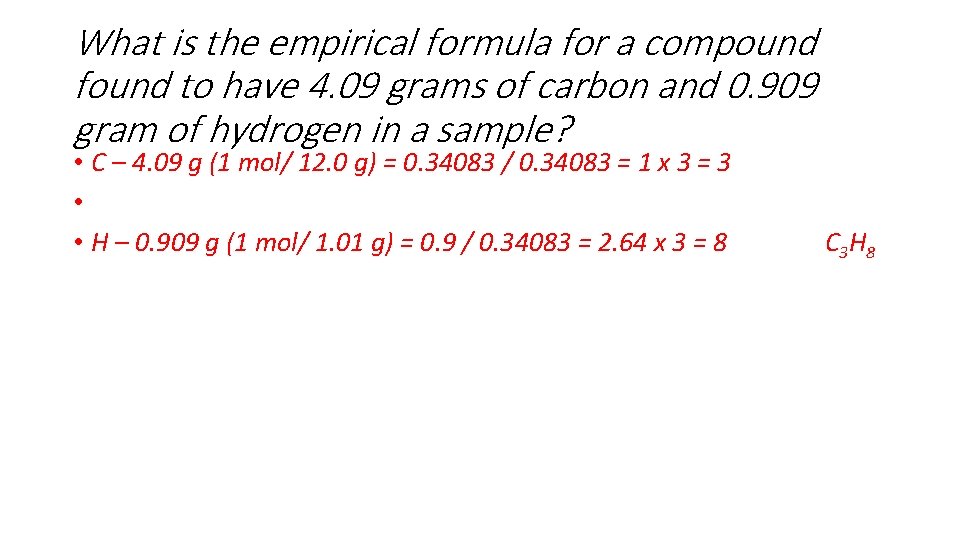
What is the empirical formula for a compound found to have 4. 09 grams of carbon and 0. 909 gram of hydrogen in a sample? • C – 4. 09 g (1 mol/ 12. 0 g) = 0. 34083 / 0. 34083 = 1 x 3 = 3 • • H – 0. 909 g (1 mol/ 1. 01 g) = 0. 9 / 0. 34083 = 2. 64 x 3 = 8 C 3 H 8


- Slides: 9