7 4 Aim How do we use moles











- Slides: 14

#7. 4 Aim: How do we use moles to count atoms? Agenda QOD (5) HW review (5) Lesson: mole conversions (25) Activity: Mole conversions (5) Closing (5) No HW Do Now: 1. What is a mole? 6. 022 x 1023 2. How many atoms are in 1 mole of carbon? 6. 022 x 1023 3. How much does one mole of carbon atoms weigh? 12. 01 grams 4. How much does 1 mole of carbon dioxide weigh? 44 grams 5. How much does 2 moles of carbon dioxide weigh? 88 grams

#7. 4 Aim: How do we use moles to count atoms? Agenda QOD (5) HW review (5) Lesson: mole conversions (25) Activity: Mole conversions (5) Closing (5) No HW Mole conversions : How do we use moles to count atoms?

#7. 4 Aim: How do we use moles to count atoms? 1 Mole = a “gazillion” Agenda QOD (5) HW review (5) Lesson: mole conversions (25) Activity: Mole conversions (5) Closing (5) No HW Where does that craaaazy big number come from? 1 mole = # of atoms in 12 g of carbon. So… 1 mole of C has a mass of 12 g… What is the mass of 3. 7 mol of C?

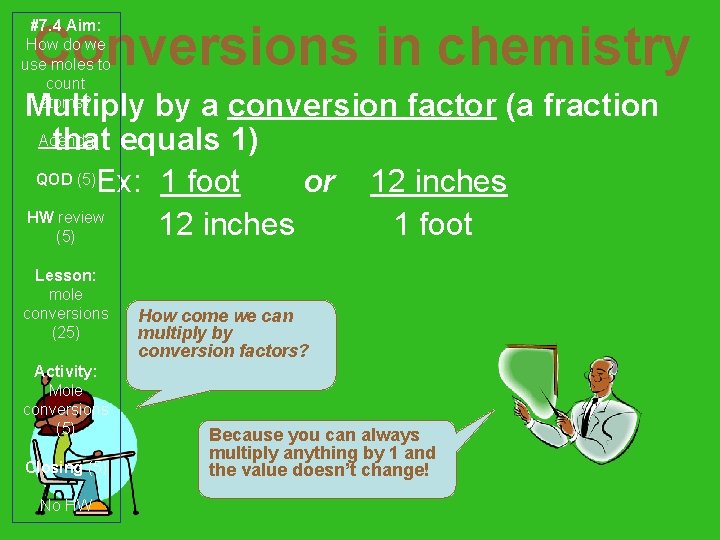
Conversions in chemistry #7. 4 Aim: How do we use moles to count atoms? Multiply by a conversion factor (a fraction Agenda that equals 1) QOD (5)Ex: 1 foot or 12 inches HW review 12 inches 1 foot (5) Lesson: mole conversions (25) Activity: Mole conversions (5) Closing (5) No HW How come we can multiply by conversion factors? Because you can always multiply anything by 1 and the value doesn’t change!

#7. 4 Aim: How do we use moles to count atoms? For instance… how many minutes are in 1 day? Agenda We know that…. QOD (5) • 60 minutes = 1 hour HW review (5) • 24 hours = 1 day Lesson: mole conversions (25) 1 day x 24 hours x 60 minutes = 1440 minutes 1 day 1 hour Activity: Mole conversions (5) Closing (5) No HW Conversion factors

Conversions in chemistry #7. 4 Aim: How do we use moles to count atoms? In chemistry we can use the conversion Agenda factor: QOD (5) molar mass (in grams) = 1 mol HW review (5) Lesson: mole conversions (25) Activity: Mole conversions (5) Closing (5) No HW

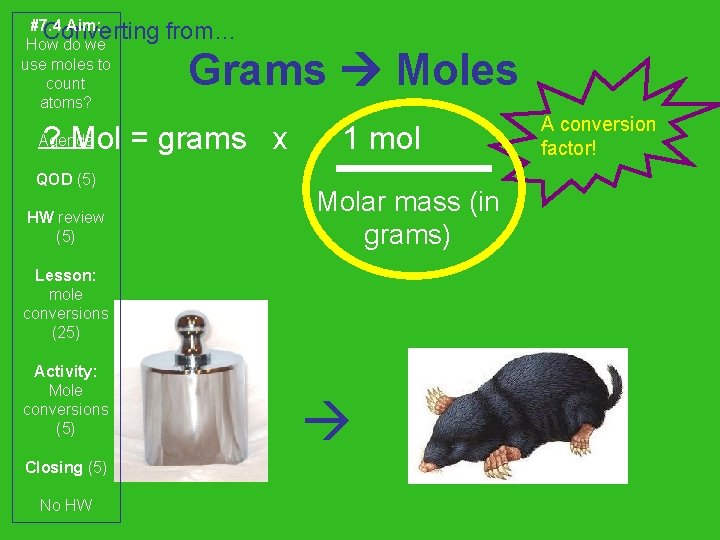
#7. 4 Aim: Converting How do we use moles to count atoms? from… Grams Moles ? Mol = grams x Agenda QOD (5) HW review (5) 1 mol Molar mass (in grams) Lesson: mole conversions (25) Activity: Mole conversions (5) Closing (5) No HW A conversion factor!

#7. 4 Aim: How do we use moles to count atoms? Example: 1 mole Agenda of Carbon is equal to 12. 01 grams. QOD (5) HW review (5) The conversion factor is: Lesson: mole conversions (25) Activity: Mole conversions (5) Closing (5) No HW 1 mol OR 12. 01 grams 1 mole

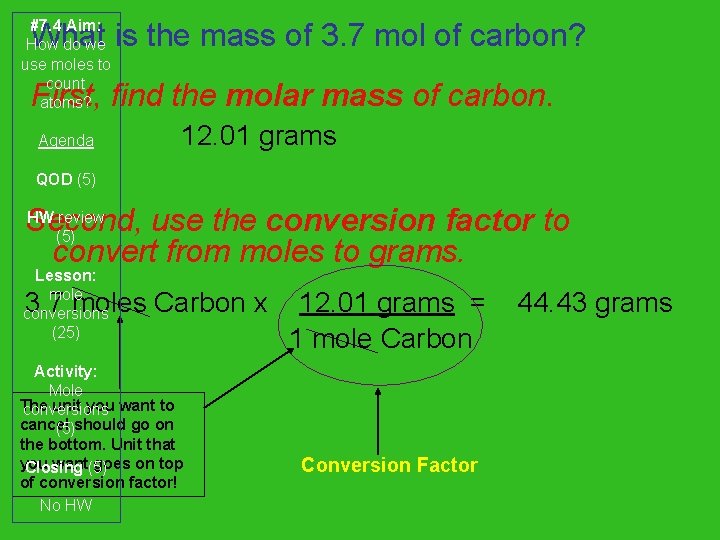
#7. 4 Aim: How do we use moles to count atoms? What is the mass of 3. 7 mol of carbon? First, find the molar mass of carbon. Agenda 12. 01 grams QOD (5) HW review Second, use the conversion factor to (5) convert from moles to grams. Lesson: mole conversions (25) 3. 7 moles Carbon x Activity: Mole The unit you want to conversions cancel (5)should go on the bottom. Unit that you want(5) goes on top Closing of conversion factor! No HW 12. 01 grams = 1 mole Carbon Conversion Factor 44. 43 grams

#7. 4 Aim: How do we use moles to count atoms? How much does 1. 32 moles of Carbon Dioxide (CO 2) weigh? First, find the molar mass of carbon dioxide. Agenda For C: 1 x (12. 01) = 12. 0 g For O: 2 x (16. 0) = 32. 00 g QOD (5) -----------------------Total: 44. 0 grams HW review (5) Lesson: Second, use the conversion factor to mole convert from moles to grams. conversions (25) 3. 7 moles CO 2 x Activity: Mole conversions (5) Closing (5) No HW 44. 0 grams = 162. 8 grams 1 mole CO 2

#7. 4 Aim: How do we use moles to count atoms? How many moles of carbon in 36. 0 grams? First, find the molar mass of Carbon. Agenda 12. 01 grams QOD (5) Second, use this conversion factor to convert HW review (5) from grams to moles. Lesson: mole conversions (25) 36 grams Carbon x 1 mole Carbon = 2. 99 moles Carbon 12. 01 grams Activity: Mole conversions (5) Closing (5) No HW Conversion Factor

#7. 4 Aim: How do we use moles to count atoms? How many moles of Na. OH in 2. 4 grams? First, find the molar mass of Na. OH. For Na: 1 x (23. 0) = 23. 0 g Agenda For O: 1 x (16. 0) = 16. 0 g For H: 1 x (1. 0) = 1. 0 g QOD (5) -----------------------HW review Total: 40. 0 grams (5) Lesson: Second, use this conversion factor to convert mole conversions from grams to moles. (25) 2. 4 g Na. OH x 1 mole Na. OH = 0. 06 moles Na. OH Activity: Mole conversions (5) 40. 0 grams Closing (5) No HW Conversion Factor

#7. 4 Aim: How do we use moles to count atoms? Agenda QOD (5) HW review (5) Lesson: mole conversions (25) Activity: Mole conversions (5) Closing (5) No HW Activity: Mole conversions Work with your group-mates to start your class-work… we’ll finish next class.

#7. 4 Aim: How do we use moles to count atoms? Agenda QOD (5) Closing: Think: to solve this problem do you divide by the gram-formula mass or multiply? HW review (5) Lesson: mole conversions (25) Activity: Mole conversions (5) Closing (5) No HW How many moles of water in 36. 0 g of water?