7 3 OxidationReduction Reactions Rust forms when the

- Slides: 13

7. 3 Oxidation−Reduction Reactions Rust forms when the oxygen in the air reacts with iron. In this process, electrons are transferred from one substance to another. Learning Goal Define the terms oxidation and reduction; identify the reactants oxidized and reduced. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Oxidation−Reduction Reactions An oxidation–reduction reaction • provides us with energy from food. • provides electrical energy in batteries. • occurs when iron rusts: 4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

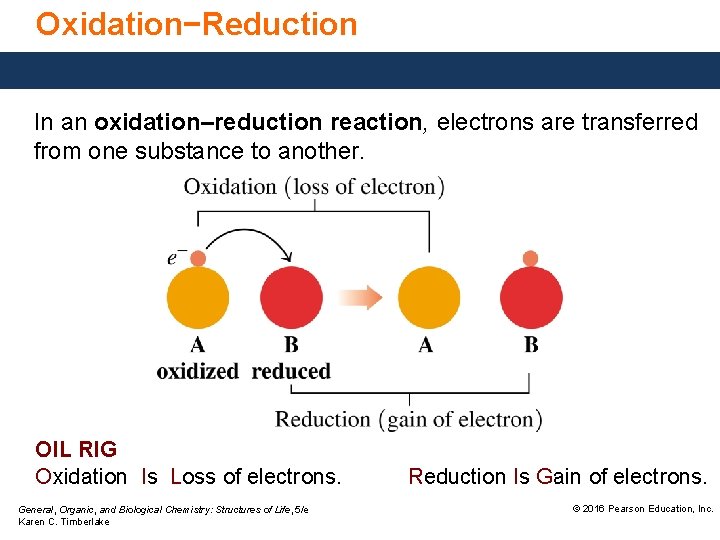
Oxidation−Reduction In an oxidation–reduction reaction, electrons are transferred from one substance to another. OIL RIG Oxidation Is Loss of electrons. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake Reduction Is Gain of electrons. © 2016 Pearson Education, Inc.

Oxidation−Reduction The green patina on the Statue of Liberty is due to the oxidation of copper metal as it forms a green solid, Cu. O. 2 Cu(s) 2 Cu 2+(s) + 4 e− oxidation O 2(g) + 4 e− 2 O 2− (s) reduction 2 Cu(s) + O 2(g) 2 Cu. O(s) Core Chemistry Skill Identifying Oxidized and Reduced Substances General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

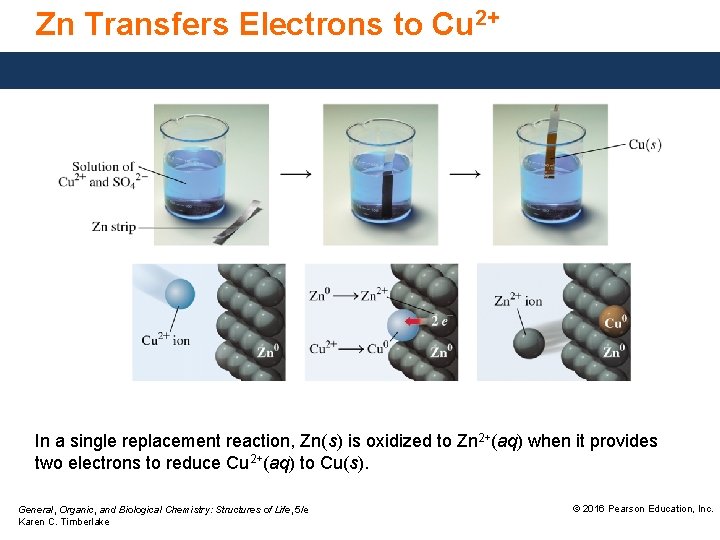
Zn Transfers Electrons to Cu 2+ In a single replacement reaction, Zn(s) is oxidized to Zn 2+(aq) when it provides two electrons to reduce Cu 2+(aq) to Cu(s). General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

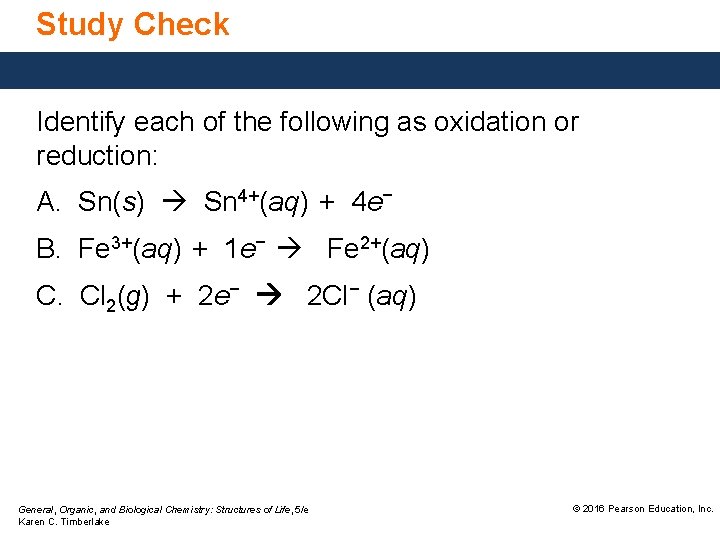
Study Check Identify each of the following as oxidation or reduction: A. Sn(s) Sn 4+(aq) + 4 e− B. Fe 3+(aq) + 1 e− Fe 2+(aq) C. Cl 2(g) + 2 e− 2 Cl− (aq) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

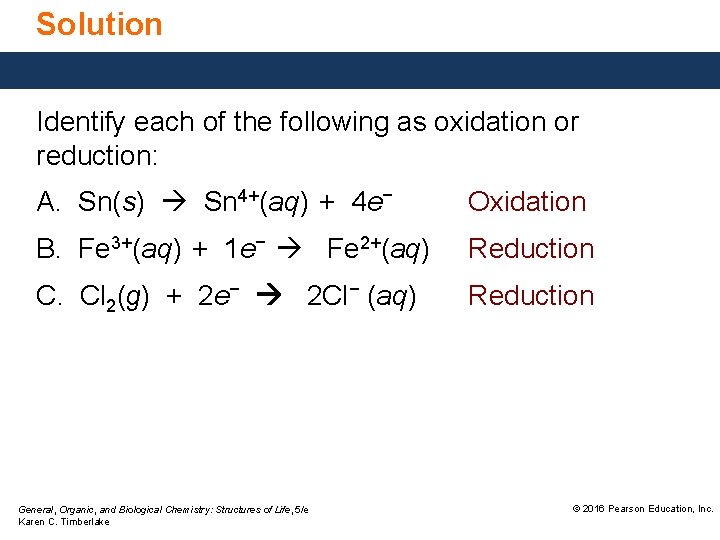
Solution Identify each of the following as oxidation or reduction: A. Sn(s) Sn 4+(aq) + 4 e− Oxidation B. Fe 3+(aq) + 1 e− Fe 2+(aq) Reduction C. Cl 2(g) + 2 e− 2 Cl− (aq) Reduction General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

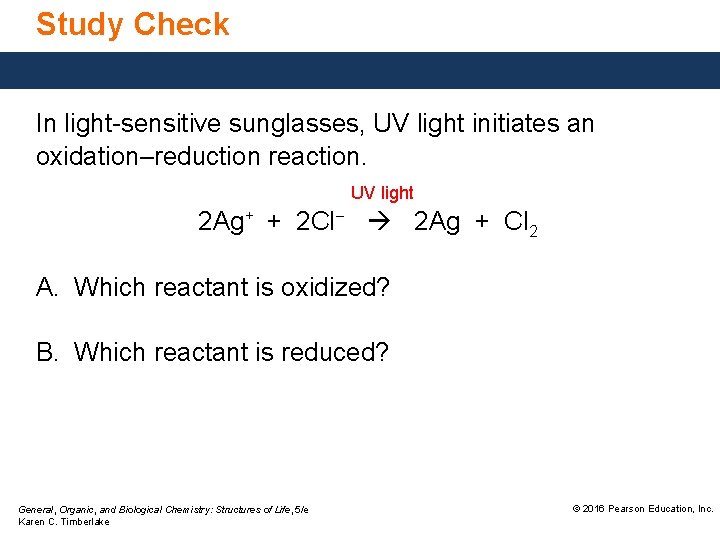
Study Check In light-sensitive sunglasses, UV light initiates an oxidation–reduction reaction. UV light 2 Ag+ + 2 Cl− 2 Ag + Cl 2 A. Which reactant is oxidized? B. Which reactant is reduced? General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

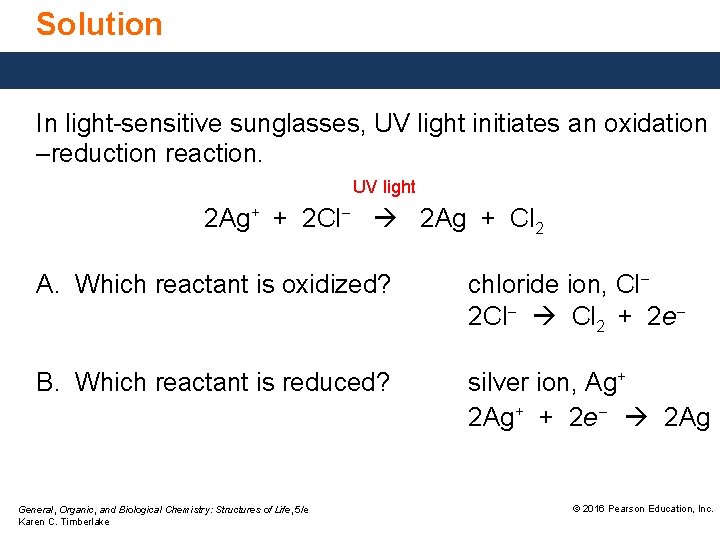
Solution In light-sensitive sunglasses, UV light initiates an oxidation –reduction reaction. UV light 2 Ag+ + 2 Cl− 2 Ag + Cl 2 A. Which reactant is oxidized? chloride ion, Cl− 2 Cl− Cl 2 + 2 e− B. Which reactant is reduced? silver ion, Ag+ 2 Ag+ + 2 e− 2 Ag General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

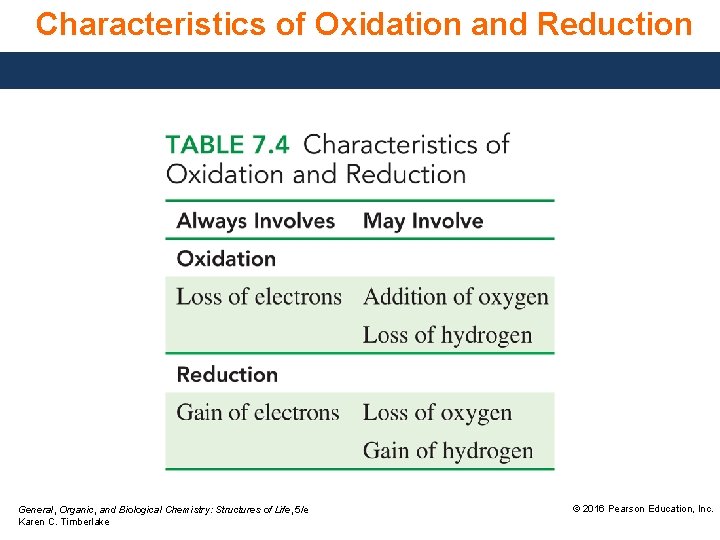
Characteristics of Oxidation and Reduction General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

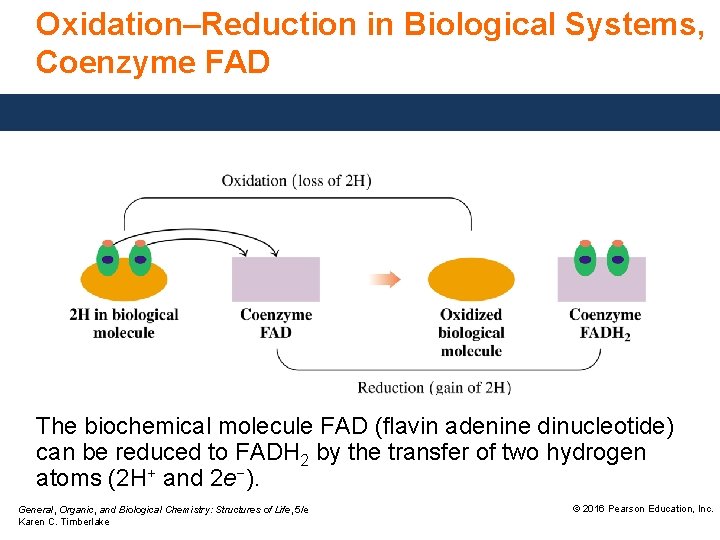
Oxidation–Reduction in Biological Systems, Coenzyme FAD The biochemical molecule FAD (flavin adenine dinucleotide) can be reduced to FADH 2 by the transfer of two hydrogen atoms (2 H+ and 2 e−). General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Oxidation–Reduction of CH 3 OH In many biochemical oxidation–reduction reactions, the transfer of hydrogen atoms is necessary for the production of energy. • For example, the body metabolizes methyl alcohol, a poisonous substance, by the following reactions: CH 3 OH H 2 CO + 2 H Oxidation: loss of H atoms methyl alcohol formaldehyde 2 H 2 CO + O 2 2 H 2 CO 2 Oxidation: addition of O atoms formaldehyde formic acid 2 H 2 CO 2 + O 2 2 CO 2 + 2 H 2 O Oxidation: addition of O atoms formic acid • The intermediate products are toxic, causing headaches and possible death because they interfere with key reactions in cells. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Characteristics, Oxidation−Reduction The particular definition of oxidation and reduction depends on the process that occurs in the reaction. Oxidation • always involves a loss of electrons. • may also be seen as an addition of oxygen. • may also be seen as the loss of hydrogen atoms. Reduction • always involves a gain of electrons. • may also be seen as the loss of oxygen. • may also be seen as the gain of hydrogen. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.