602 000 000 000 Stoichiometry Math of Chemistry







- Slides: 17

602, 000, 000, 000 Stoichiometry Math of Chemistry 1

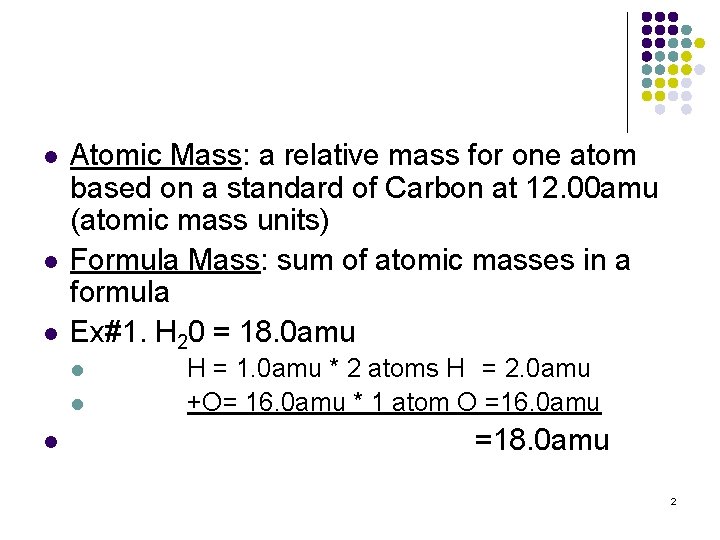
l l l Atomic Mass: a relative mass for one atom based on a standard of Carbon at 12. 00 amu (atomic mass units) Formula Mass: sum of atomic masses in a formula Ex#1. H 20 = 18. 0 amu l l l H = 1. 0 amu * 2 atoms H = 2. 0 amu +O= 16. 0 amu * 1 atom O =16. 0 amu =18. 0 amu 2

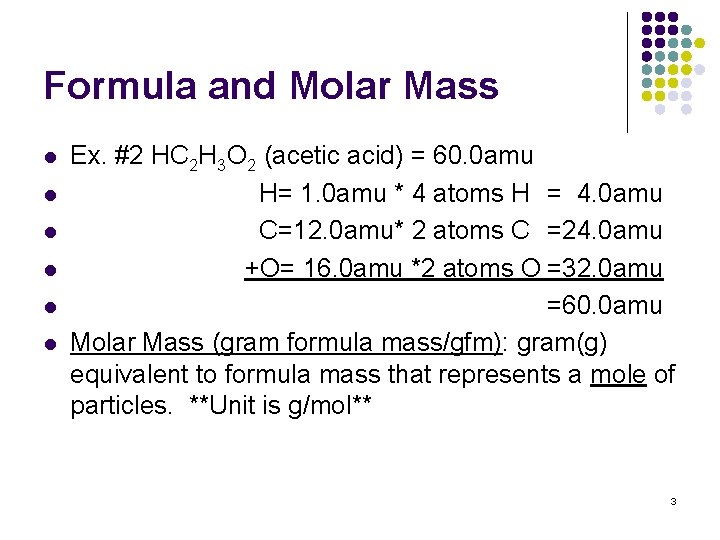
Formula and Molar Mass l l l Ex. #2 HC 2 H 3 O 2 (acetic acid) = 60. 0 amu H= 1. 0 amu * 4 atoms H = 4. 0 amu C=12. 0 amu* 2 atoms C =24. 0 amu +O= 16. 0 amu *2 atoms O =32. 0 amu =60. 0 amu Molar Mass (gram formula mass/gfm): gram(g) equivalent to formula mass that represents a mole of particles. **Unit is g/mol** 3

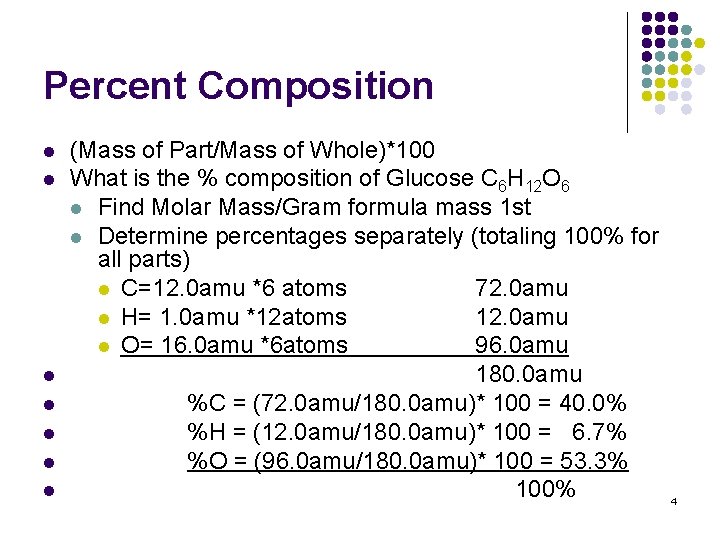
Percent Composition l l l l (Mass of Part/Mass of Whole)*100 What is the % composition of Glucose C 6 H 12 O 6 l Find Molar Mass/Gram formula mass 1 st l Determine percentages separately (totaling 100% for all parts) l C=12. 0 amu *6 atoms 72. 0 amu l H= 1. 0 amu *12 atoms 12. 0 amu l O= 16. 0 amu *6 atoms 96. 0 amu 180. 0 amu %C = (72. 0 amu/180. 0 amu)* 100 = 40. 0% %H = (12. 0 amu/180. 0 amu)* 100 = 6. 7% %O = (96. 0 amu/180. 0 amu)* 100 = 53. 3% 100% 4

The Mole and Avogadro’s Number 6. 02*1023 l Avogadro’s number 6. 02* 1023 particles (atoms, molecules, formula units) equals one mole l l One mole of Lead(Pb) would have a molar mass of 207. 2 g/mol, while a mole of Carbon(C) would be 12. 0 g/mol Both 1 mole of Pb and C would have 6. 02* 1023 atoms each (or any other element as well) 5

Molecules and Random Facts l l Molecules: are considered to be a whole unit Ex. 6. 02* 1023 molecules of H 20 in a mole of water 6. 02* 1023 molecules of H 2 in a mole of hydrogen gas Random Avogadro facts: l If you had a mole of pennies, you could give out a million dollars a day for 3000 years l a mole of paper would be stacked beyond our solar system 6

Formula Units (ionic Compounds) l l Formula Unit: lowest whole # ratio of an Ionic compound 6. 02* 1023 formula units in a mole of Na. Cl l broken down into 2 moles of ions l l 1 mole Na+ ion 1 mole Cl- ion 6. 02* 1023 formula units in a mole of Ca. Cl 2 l broken down into 3 moles of ions l 1 mole Ca+2 ion 2 moles Cl- ion 7

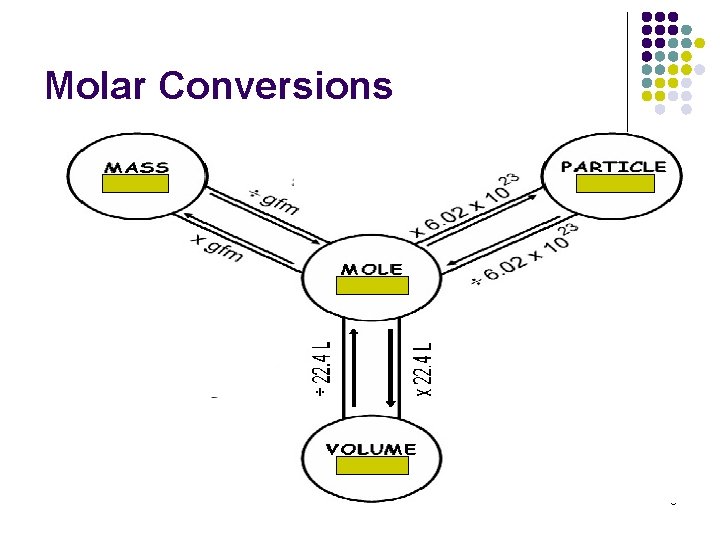
Molar Conversions 8

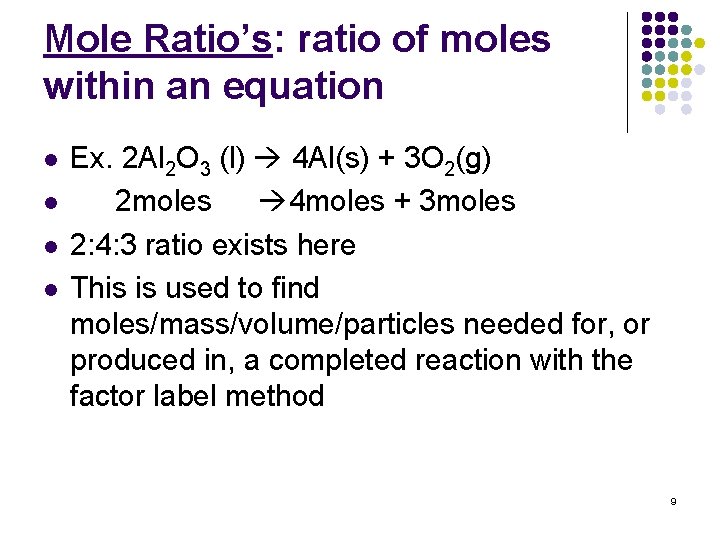
Mole Ratio’s: ratio of moles within an equation l l Ex. 2 Al 2 O 3 (l) 4 Al(s) + 3 O 2(g) 2 moles 4 moles + 3 moles 2: 4: 3 ratio exists here This is used to find moles/mass/volume/particles needed for, or produced in, a completed reaction with the factor label method 9

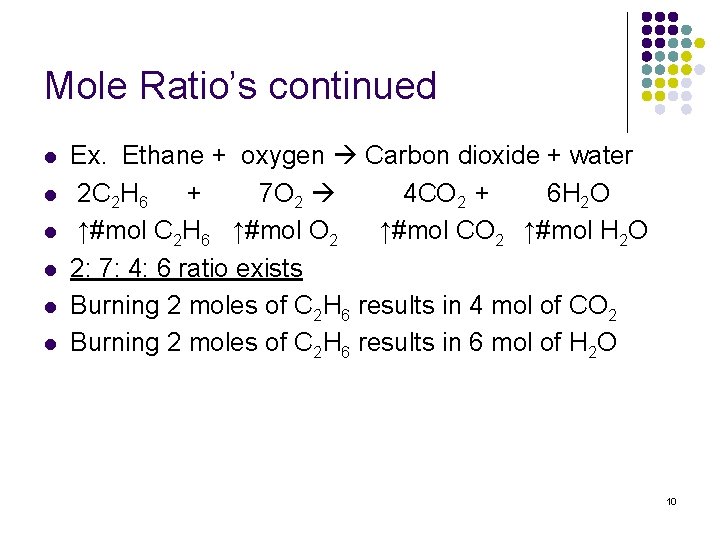
Mole Ratio’s continued l l l Ex. Ethane + oxygen Carbon dioxide + water 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O ↑#mol C 2 H 6 ↑#mol O 2 ↑#mol CO 2 ↑#mol H 2 O 2: 7: 4: 6 ratio exists Burning 2 moles of C 2 H 6 results in 4 mol of CO 2 Burning 2 moles of C 2 H 6 results in 6 mol of H 2 O 10

Mole Ratio’s continued l 2 C 2 H 6 l Ex#1 How many moles of water are produced from the combustion of 3 mol of ethane gas? (3 mol C 2 H 6) (6 mol H 2 O/2 mol C 2 H 6) = 9 mol H 2 O l l l + 7 O 2 4 CO 2 + 6 H 2 O Ex #2 If 5 moles of ethane are burned, how much carbon dioxide is produced? (5 mol C 2 H 6)(4 mol CO 2 /2 mol C 2 H 6) = 10 mol CO 2 11

Empirical Formula l Simplest/lowest whole number ratio of elements in a compound or molecule. Ex. Glucose C 6 H 12 O 6 CH 2 O l Ex. C 54 H 110 would be what? l 12

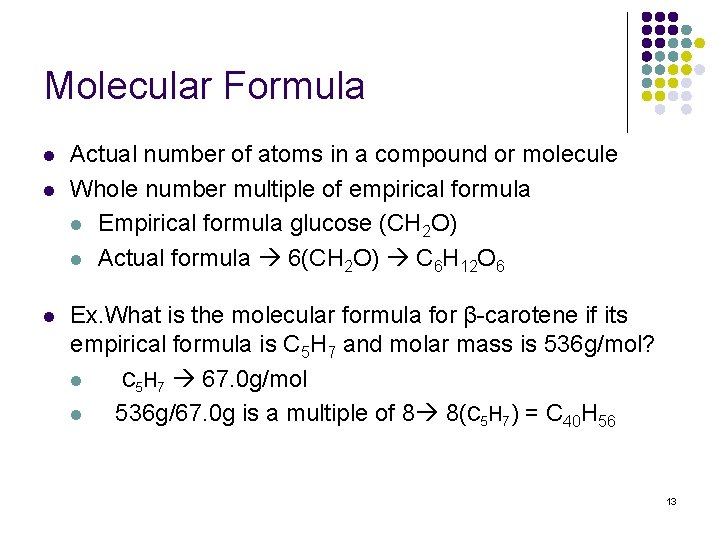
Molecular Formula l l l Actual number of atoms in a compound or molecule Whole number multiple of empirical formula l Empirical formula glucose (CH 2 O) l Actual formula 6(CH 2 O) C 6 H 12 O 6 Ex. What is the molecular formula for β-carotene if its empirical formula is C 5 H 7 and molar mass is 536 g/mol? l C 5 H 7 67. 0 g/mol l 536 g/67. 0 g is a multiple of 8 8(C 5 H 7) = C 40 H 56 13

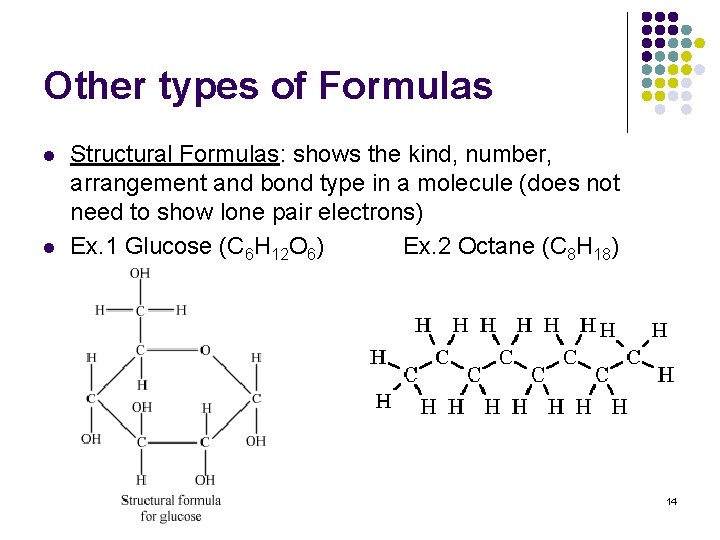
Other types of Formulas l l Structural Formulas: shows the kind, number, arrangement and bond type in a molecule (does not need to show lone pair electrons) Ex. 1 Glucose (C 6 H 12 O 6) Ex. 2 Octane (C 8 H 18) 14

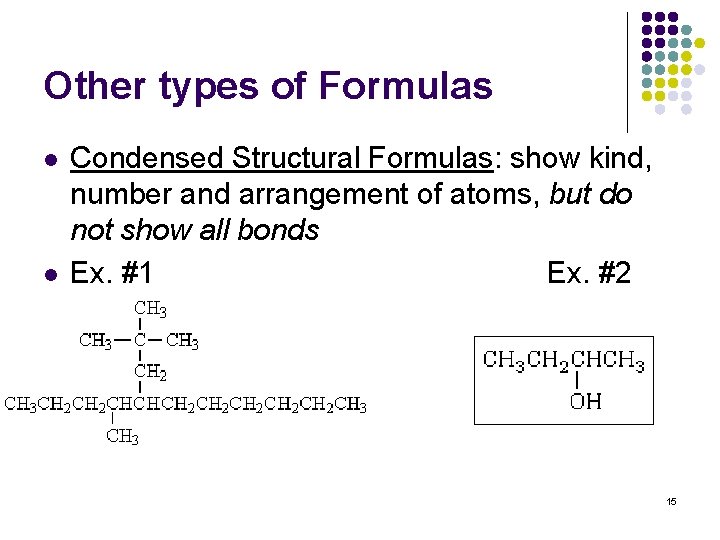
Other types of Formulas l l Condensed Structural Formulas: show kind, number and arrangement of atoms, but do not show all bonds Ex. #1 Ex. #2 15

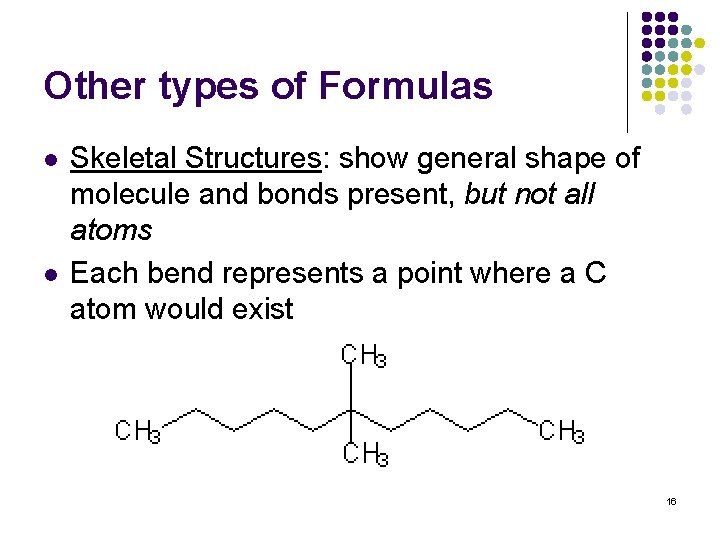
Other types of Formulas l l Skeletal Structures: show general shape of molecule and bonds present, but not all atoms Each bend represents a point where a C atom would exist 16

Types of Chemical Reactions and Equations l Word equation l l Formula equation l l One mole of Methane and two moles of oxygen yield a mole of carbon dioxide and two moles of water CH 4 + 202 CO 2 and 2 H 2 O Reversible reaction l Represented by a double arrow l ↔ or 17
 602 200 000 000 000 000 000 000 in scientific notation
602 200 000 000 000 000 000 000 in scientific notation 97 700 000 000 000 000 000 000 in scientific notation
97 700 000 000 000 000 000 000 in scientific notation 33 900 000 in scientific notation
33 900 000 in scientific notation Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Chapter 9 review stoichiometry
Chapter 9 review stoichiometry Ap chemistry stoichiometry
Ap chemistry stoichiometry Reaction stoichiometry definition
Reaction stoichiometry definition Chapter 11 study guide chemistry stoichiometry answer key
Chapter 11 study guide chemistry stoichiometry answer key Chapter 9 stoichiometry answer key
Chapter 9 stoichiometry answer key General chemistry 1 stoichiometry
General chemistry 1 stoichiometry Ap chemistry stoichiometry
Ap chemistry stoichiometry 090-0000-0000
090-0000-0000 Kamstrup multical 802
Kamstrup multical 802 602 x 10
602 x 10 Ece 602 purdue
Ece 602 purdue Kreis 602
Kreis 602 E=1 602*10
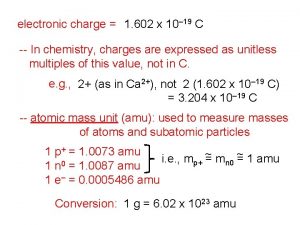
E=1 602*10 1 602 x 10^-19
1 602 x 10^-19