6 Fluorescence Spectroscopy 1 IPC FriedrichSchillerUniversitt Jena Rotational
![Rotational levels v = 1 v = 0 Excitation [10 -15 s] 6. Basic Rotational levels v = 1 v = 0 Excitation [10 -15 s] 6. Basic](https://slidetodoc.com/presentation_image_h/78eed37219fb0ea9c81b19f1547e872b/image-2.jpg)

- Slides: 18

6. Fluorescence Spectroscopy 1 IPC Friedrich-Schiller-Universität Jena
![Rotational levels v 1 v 0 Excitation 10 15 s 6 Basic Rotational levels v = 1 v = 0 Excitation [10 -15 s] 6. Basic](https://slidetodoc.com/presentation_image_h/78eed37219fb0ea9c81b19f1547e872b/image-2.jpg)
Rotational levels v = 1 v = 0 Excitation [10 -15 s] 6. Basic concepts in fluorescence spectroscopy J = 4 Microwavespectroscopy J = 3 J = 2 J = 1 J = 0 UV-VIS-spectroscopy S 4 S 3 Internal conversion [10 -14 s] Tn S 2 IR- & NIRspectroscopy Intersystem crossing v = 4 v = 3 Vibrational levels S 1 T 1 Fluorescence Phosphorescence [10 -9 s] [10 -3 s] v = 1 v = 0 2 S 0 IPC Friedrich-Schiller-Universität Jena

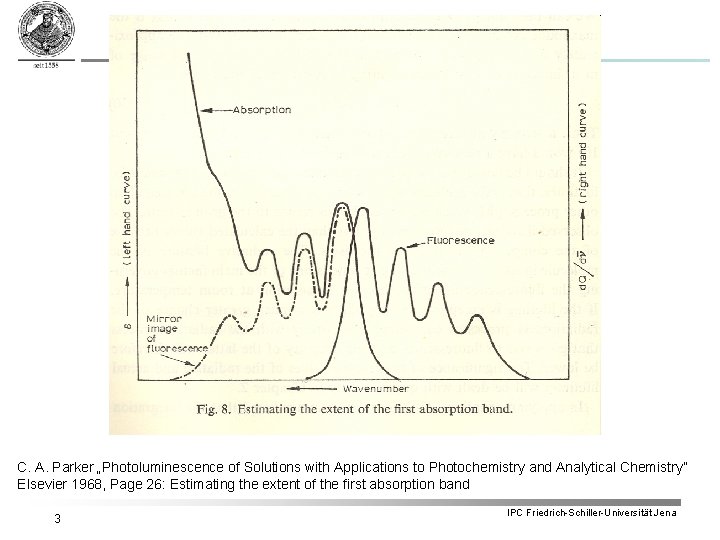
C. A. Parker „Photoluminescence of Solutions with Applications to Photochemistry and Analytical Chemistry” Elsevier 1968, Page 26: Estimating the extent of the first absorption band 3 IPC Friedrich-Schiller-Universität Jena

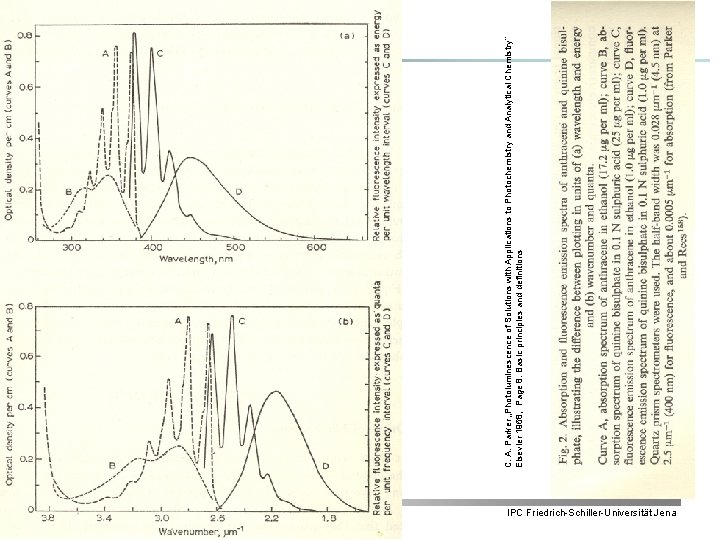
4 IPC Friedrich-Schiller-Universität Jena C. A. Parker „Photoluminescence of Solutions with Applications to Photochemistry and Analytical Chemistry” Elsevier 1968, Page 8: Basic principles and definitions

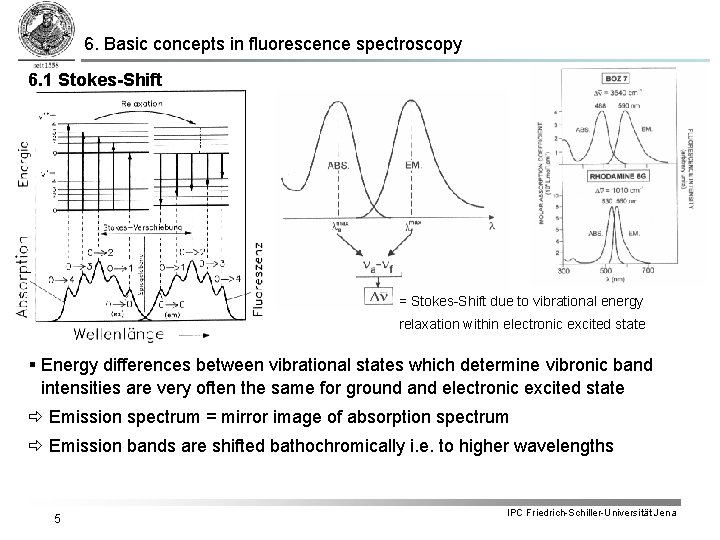
6. Basic concepts in fluorescence spectroscopy 6. 1 Stokes-Shift = Stokes-Shift due to vibrational energy relaxation within electronic excited state § Energy differences between vibrational states which determine vibronic band intensities are very often the same for ground and electronic excited state ð Emission spectrum = mirror image of absorption spectrum ð Emission bands are shifted bathochromically i. e. to higher wavelengths 5 IPC Friedrich-Schiller-Universität Jena

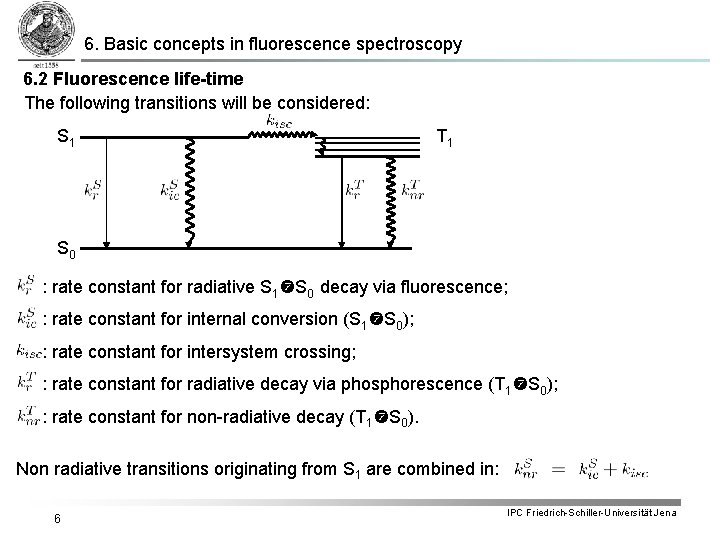
6. Basic concepts in fluorescence spectroscopy 6. 2 Fluorescence life-time The following transitions will be considered: S 1 T 1 S 0 : rate constant for radiative S 1 S 0 decay via fluorescence; : rate constant for internal conversion (S 1 S 0); : rate constant for intersystem crossing; : rate constant for radiative decay via phosphorescence (T 1 S 0); : rate constant for non-radiative decay (T 1 S 0). Non radiative transitions originating from S 1 are combined in: 6 IPC Friedrich-Schiller-Universität Jena

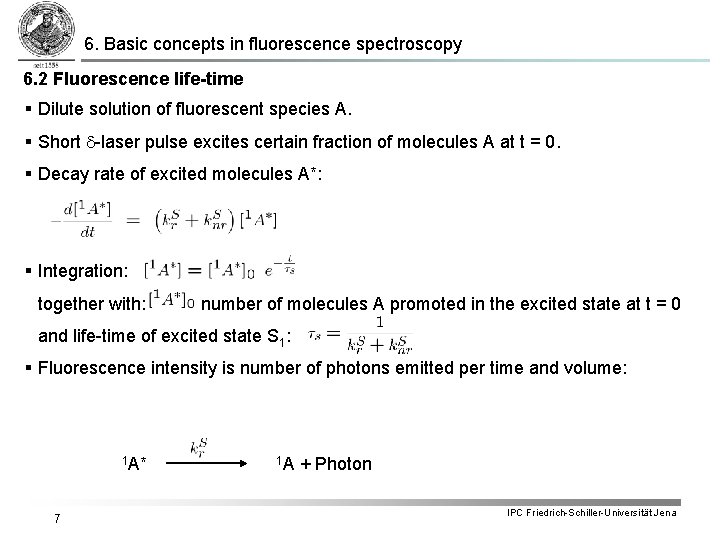
6. Basic concepts in fluorescence spectroscopy 6. 2 Fluorescence life-time § Dilute solution of fluorescent species A. § Short d-laser pulse excites certain fraction of molecules A at t = 0. § Decay rate of excited molecules A*: § Integration: together with: number of molecules A promoted in the excited state at t = 0 and life-time of excited state S 1: § Fluorescence intensity is number of photons emitted per time and volume: 1 A* 7 1 A + Photon IPC Friedrich-Schiller-Universität Jena

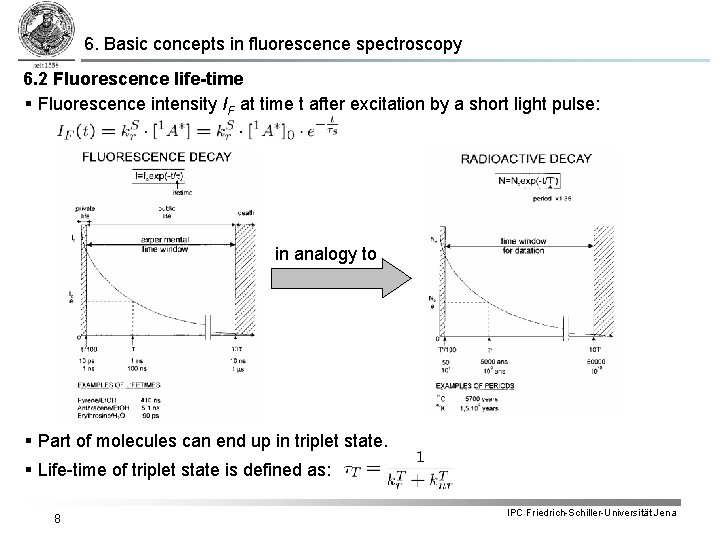
6. Basic concepts in fluorescence spectroscopy 6. 2 Fluorescence life-time § Fluorescence intensity IF at time t after excitation by a short light pulse: in analogy to § Part of molecules can end up in triplet state. § Life-time of triplet state is defined as: 8 IPC Friedrich-Schiller-Universität Jena

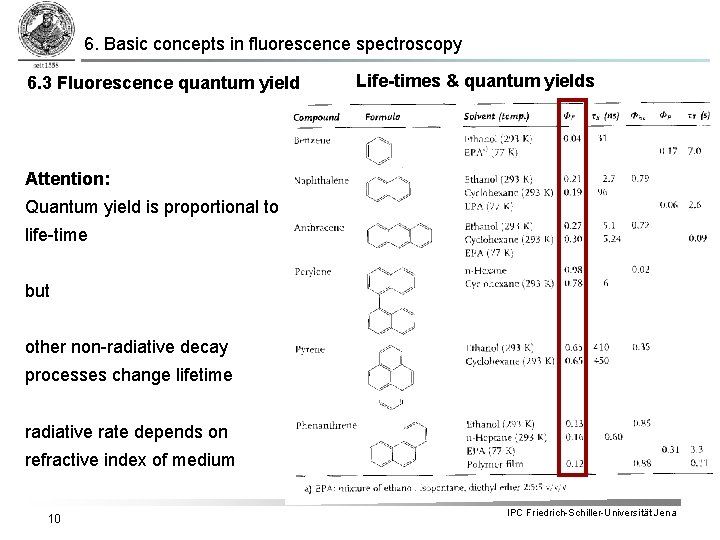
6. Basic concepts in fluorescence spectroscopy 6. 3 Fluorescence quantum yield § Fluorescence quantum yield: Emitted Photons per Excitation events § It follows: Integration over complete decay § The quantum yields for ISC and phosphorescence can be expressed in analogy: bzw. 9 IPC Friedrich-Schiller-Universität Jena

6. Basic concepts in fluorescence spectroscopy 6. 3 Fluorescence quantum yield Life-times & quantum yields Attention: Quantum yield is proportional to life-time but other non-radiative decay processes change lifetime radiative rate depends on refractive index of medium 10 IPC Friedrich-Schiller-Universität Jena

6. Basic concepts in fluorescence spectroscopy Complication 1: • Timescale of photon absorption process? • Rate of absorption is proportional to Intensity • Vibrational depopulation vs Phase decoherence time vs. Rabi Oscillations • For practical purposes (in Water): Absorbtion is faster than decoherence • Typical timescale: fs = 10 -15 s 11 IPC Friedrich-Schiller-Universität Jena

6. Basic concepts in fluorescence spectroscopy Blackboard exercise: Cross section s and absorption coefficient e 12 IPC Friedrich-Schiller-Universität Jena

6. Basic concepts in fluorescence spectroscopy Blackboard exercise: Steady State Fluorescence Saturation 13 IPC Friedrich-Schiller-Universität Jena

6. Basic concepts in fluorescence spectroscopy Complication 2: Plotting "intensity": A Mess should be called irradiance: intensity is irradiance per unit angle Units of irradiance are W/cm 2=J/(s cm 2) Photon number is proportional to irradiance !? But conversion depends on energy, wavelength: I(n) = (photons/Area/Time) • hn = (photons/Area/Time) • hc/l When plotting I(l) : measured energy flux per unit energy range DE? measured energy flux per unit slit width? measured energy flux per constant wavelength range? counted photons per unit energy range? counted photons per unit slit width? IPC Friedrich-Schiller-Universität Jena 14 counted photons per unit wavelength range?

6. Basic concepts in fluorescence spectroscopy 6. 4 Steady-State fluorescence emission § It is advantageous to define the steady-state fluorescence per absorbed photon as photon flux in dependence of wavelength (Photon spectrum) : § Emission photon spectrum expresses the probability distribution of the different transitions from the vibrational ground state of S 1 down to the various vibrational states of S 0. § The normalized steady-state fluorescence IF(l. F), recorded for the wavelength l. F is proportional to as well as to the number of absorbed photons at the excitation wavelength l. E. § Number of absorbed photons is given by: Intensity transmitted irradiated 15 IPC Friedrich-Schiller-Universität Jena

6. Basic concepts in fluorescence spectroscopy 6. 4 Steady-State fluorescence emission § Fluorescence intensity can be expressed as follows: with k = proportionality constant dependent on numerous experimental values like e. g. collection angle, band width of monochromator, slid width, etc. § Considering the intensity of the transmitted light by Lambert-Beer‘s law yields: § Recording the intensity IF as function of the wavelength l. F for a fixed excitation wavelength l. E yields fluorescence spectrum. § For low concentrations it follows: § Higher terms can be neglected for diluted solutions. § Thus it follows: A(l. E) = absorbance at l. E § Proportionality between fluorescence intensity and concentration for diluted solutions only 16 IPC Friedrich-Schiller-Universität Jena

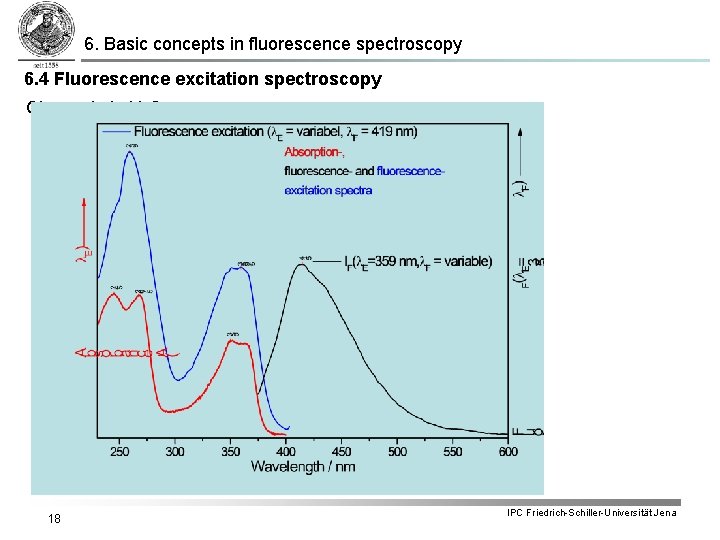
6. Basic concepts in fluorescence spectroscopy 6. 4 Fluorescence excitation spectroscopy § Recording fluorescence intensity as function of excitation wavelength l. E for a fixed observation wavelength l. F yields fluorescence excitation spectrum. § According to: the fluorescence intensity recorded as a function of the excitation wavelength reflects the product § In case the wavelength dependency of the incoming light can be compensated the fluorescence excitation spectrum depends only on what corresponds to the absorption spectrum. § As long as only one ground state species exists the corrected excitation spectrum is identical to the absorption spectrum. Otherwise a comparison between fluorescence excitation and absorption spectrum yields valuable information about the sample species present. 17 IPC Friedrich-Schiller-Universität Jena

6. Basic concepts in fluorescence spectroscopy 6. 4 Fluorescence excitation spectroscopy Cinoxacin in H 2 O 18 IPC Friedrich-Schiller-Universität Jena
 Blackboard schiller
Blackboard schiller Application of fluorescence spectroscopy
Application of fluorescence spectroscopy Principle of fluorescence spectroscopy
Principle of fluorescence spectroscopy Principles of fluorescence spectroscopy
Principles of fluorescence spectroscopy Xrf theory ppt
Xrf theory ppt Atomic fluorescence spectroscopy principle
Atomic fluorescence spectroscopy principle Rotational spectroscopy
Rotational spectroscopy Rotational spectroscopy
Rotational spectroscopy Gross selection rule for rotational spectroscopy
Gross selection rule for rotational spectroscopy Rotational spectroscopy notes
Rotational spectroscopy notes Application of rotational spectroscopy
Application of rotational spectroscopy Rotational equilibrium
Rotational equilibrium Rotational equilibrium example problems
Rotational equilibrium example problems Metu class
Metu class Guus kroonen
Guus kroonen Jena eclipse
Jena eclipse _.jenannn
_.jenannn Rainer heintzmann jena
Rainer heintzmann jena Btz jena
Btz jena