6 Electron Structures and the Periodic Table 1
6 Electron Structures and the Periodic Table 1

In 1869 Dimitri Mendeleev of Russia and Lothar Meyer of Germany independently published periodic arrangements of the elements based on increasing atomic masses. Mendeleev’s arrangement is the precursor to the modern periodic table. 2
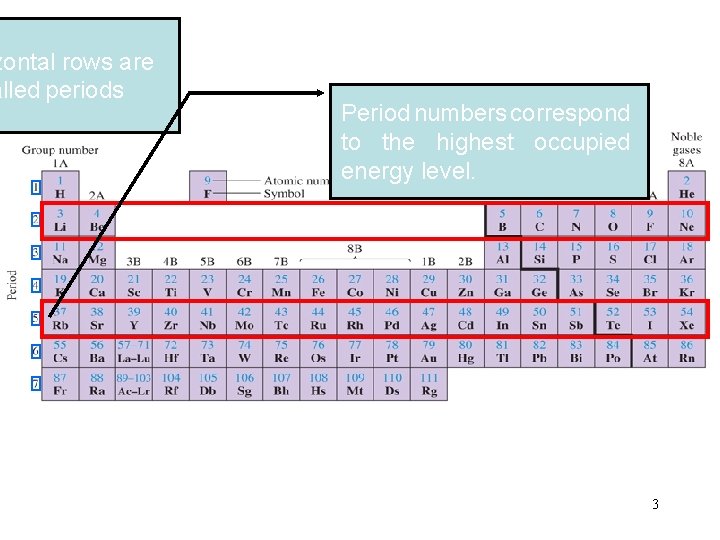
zontal rows are alled periods Period numbers correspond to the highest occupied energy level. 3
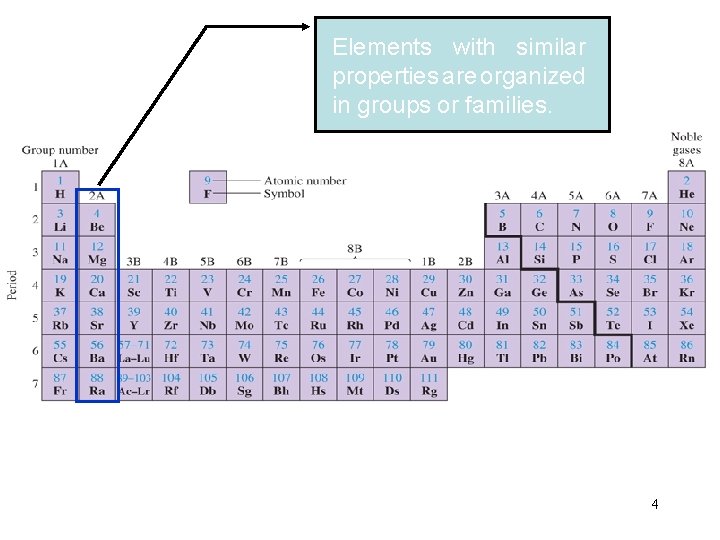
Elements with similar properties are organized in groups or families. 4
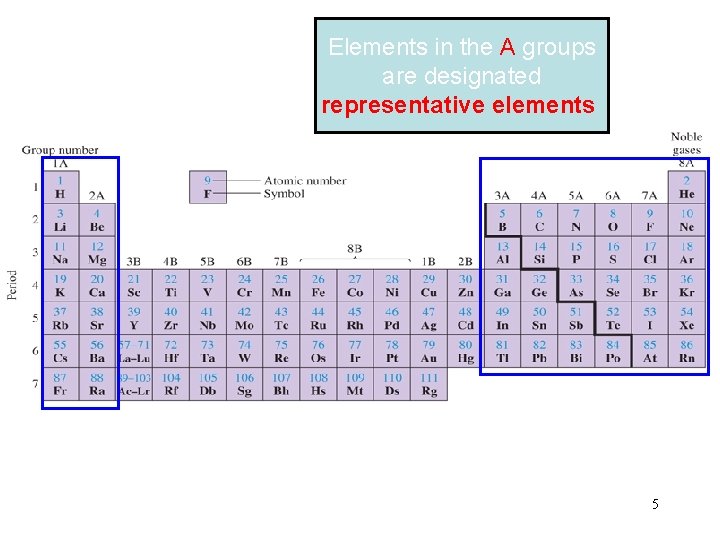
Elements in the A groups are designated representative elements 5
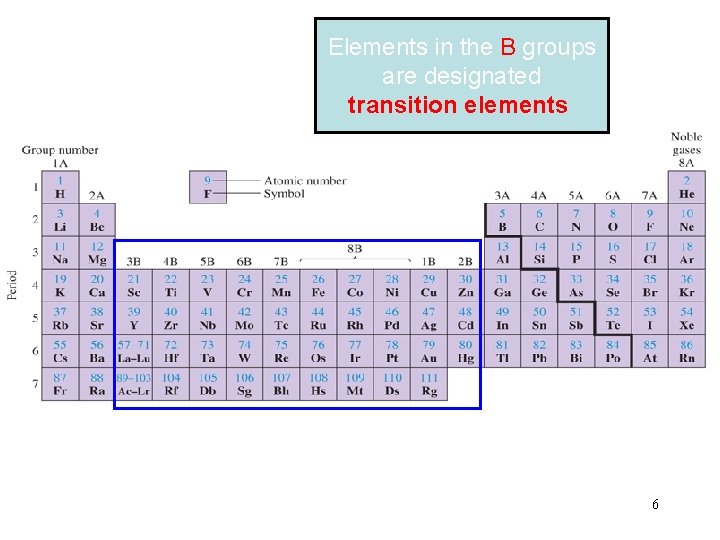
Elements in the B groups are designated transition elements 6
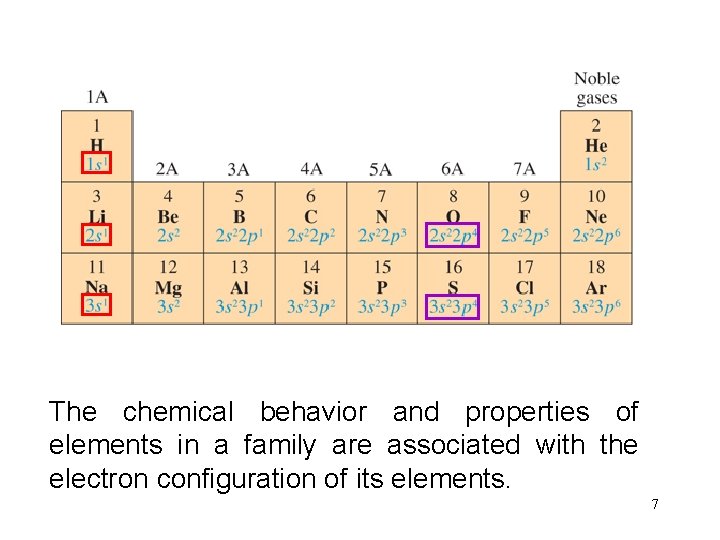
The. Forchemical A family elements behaviortheand valence properties electron of elements configuration in a family is the are same associated in each column. with the electron configuration of its elements. 7
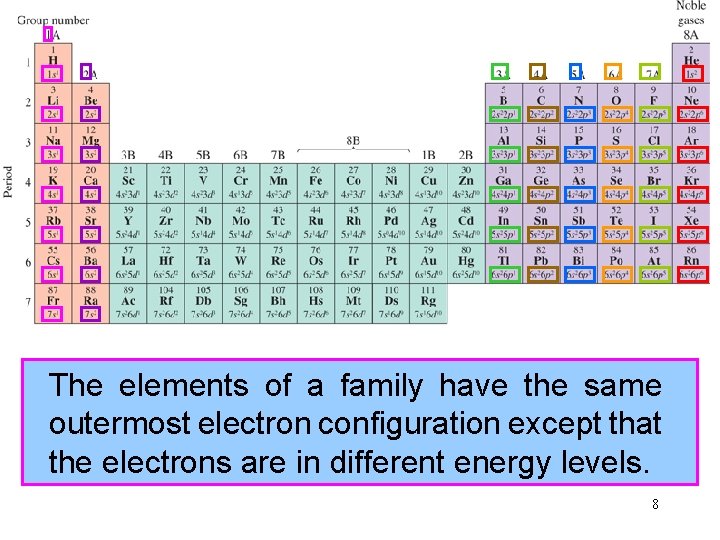
The group elements numbers of a family for thehave representative the same elements outermostare electron equalconfiguration to the total except numberthat of outermost the electrons are in in different the atoms energy of the levels. group. 8
Chemical Bonding The Formation of Compounds From Atoms
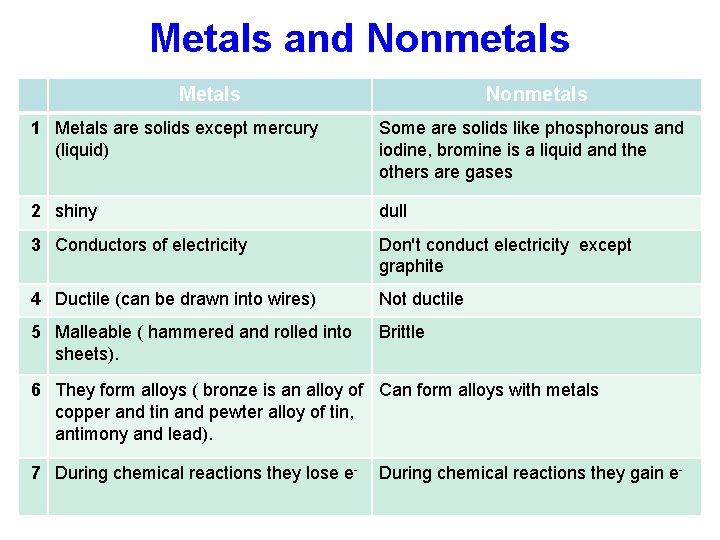
Metals and Nonmetals Metals Nonmetals 1 Metals are solids except mercury (liquid) Some are solids like phosphorous and iodine, bromine is a liquid and the others are gases 2 shiny dull 3 Conductors of electricity Don't conduct electricity except graphite 4 Ductile (can be drawn into wires) Not ductile 5 Malleable ( hammered and rolled into sheets). Brittle 6 They form alloys ( bronze is an alloy of Can form alloys with metals copper and tin and pewter alloy of tin, antimony and lead). 7 During chemical reactions they lose e- During chemical reactions they gain e-

The Metalloids have properties that are intermediate between metals and nonmetals. 1. boron 2. silicon 3. germanium 4. arsenic 5. antimony 6. tellurium 7. polonium 11
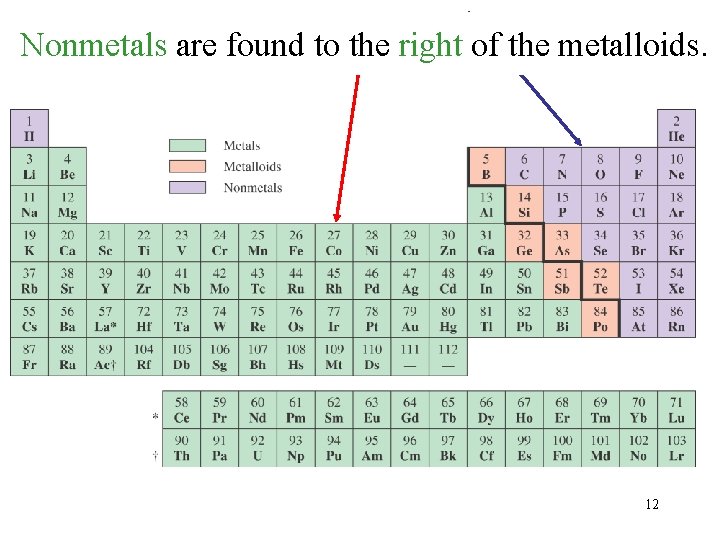
Metals areare found of of thethe metalloids Nonmetals foundtotothe theleft right metalloids. 12
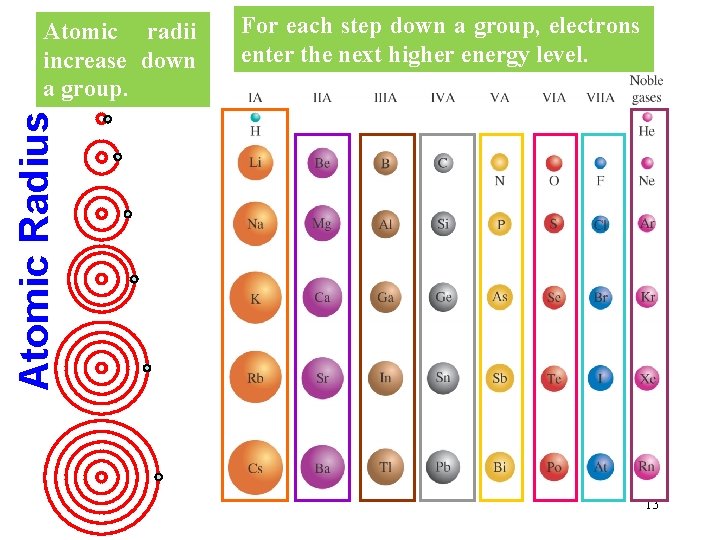
For each step down a group, electrons enter the next higher energy level. Atomic Radius Atomic radii increase down a group. 13
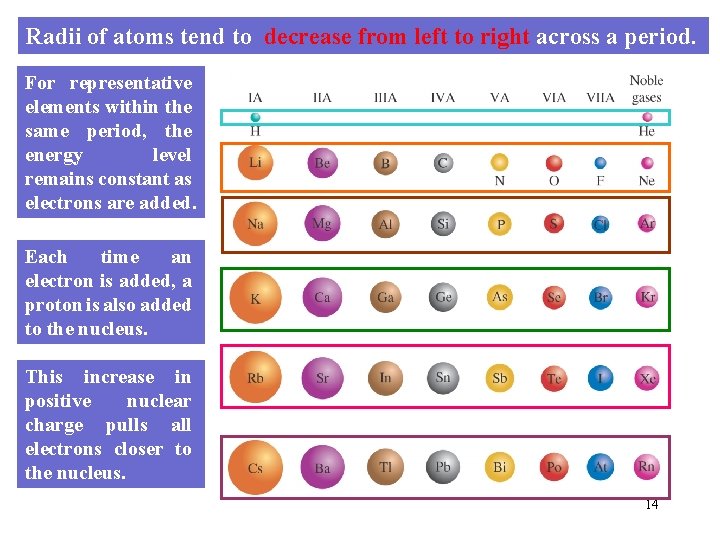
Radii of atoms tend to decrease from left to right across a period. For representative elements within the same period, the energy level remains constant as electrons are added. Each time an electron is added, a proton is also added to the nucleus. This increase in positive nuclear charge pulls all electrons closer to the nucleus. 14
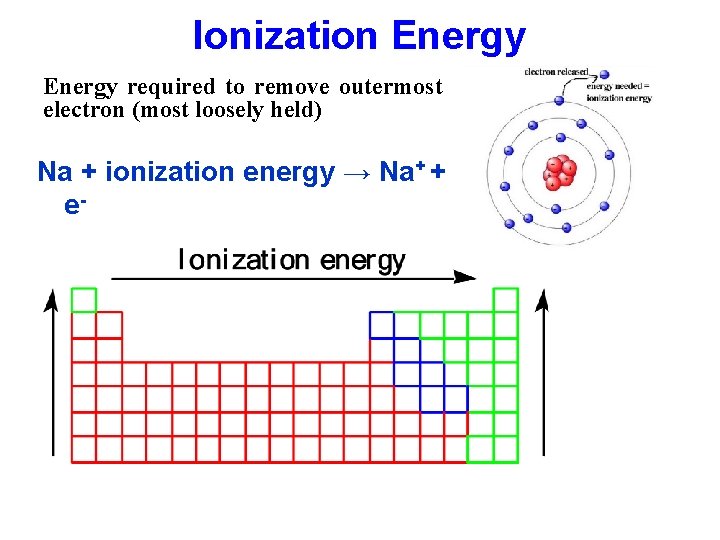
Ionization Energy required to remove outermost electron (most loosely held) Na + ionization energy → Na+ + e-
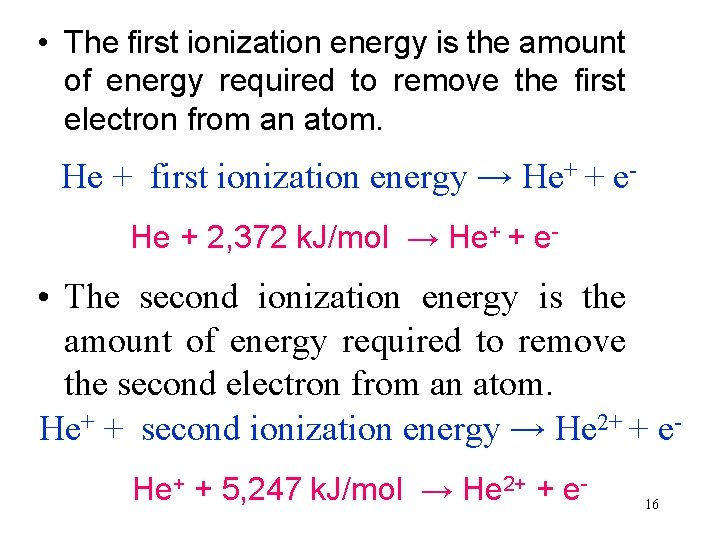
• The first ionization energy is the amount of energy required to remove the first electron from an atom. He + first ionization energy → He+ + e. He + 2, 372 k. J/mol → He+ + e- • The second ionization energy is the amount of energy required to remove the second electron from an atom. He+ + second ionization energy → He 2+ + e. He+ + 5, 247 k. J/mol → He 2+ + e- 16

The Ionic Bond Transfer of Electrons From One Atom to Another 17
The chemistry of many elements, especially the representative ones, is to attain the same outer electron structure as one of the noble gases. 18
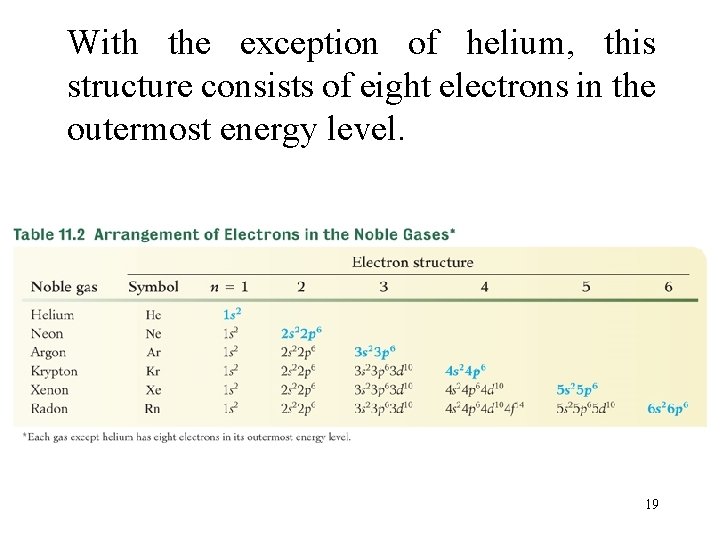
With the exception of helium, this structure consists of eight electrons in the outermost energy level. 19
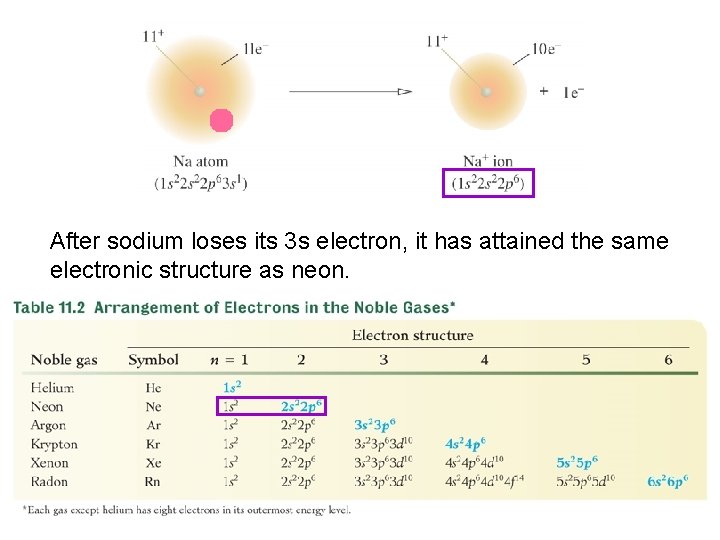
After sodium loses its 3 s electron, it has attained the same electronic structure as neon. 20
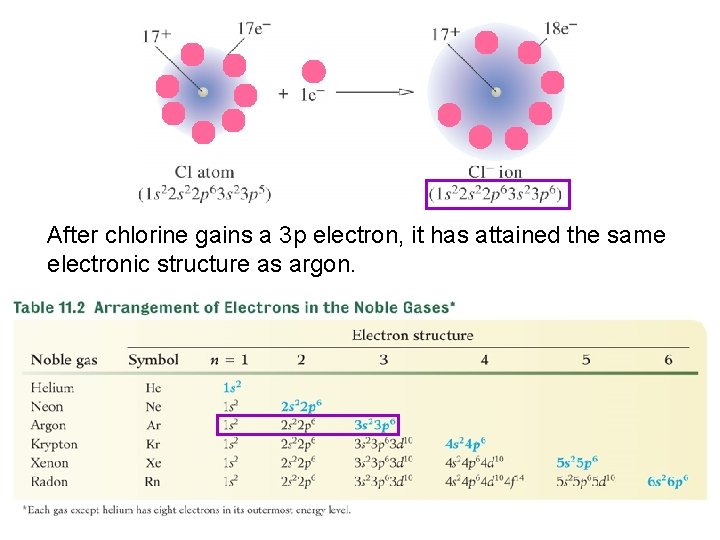
After chlorine gains a 3 p electron, it has attained the same electronic structure as argon. 21

Formation of Na. Cl 22
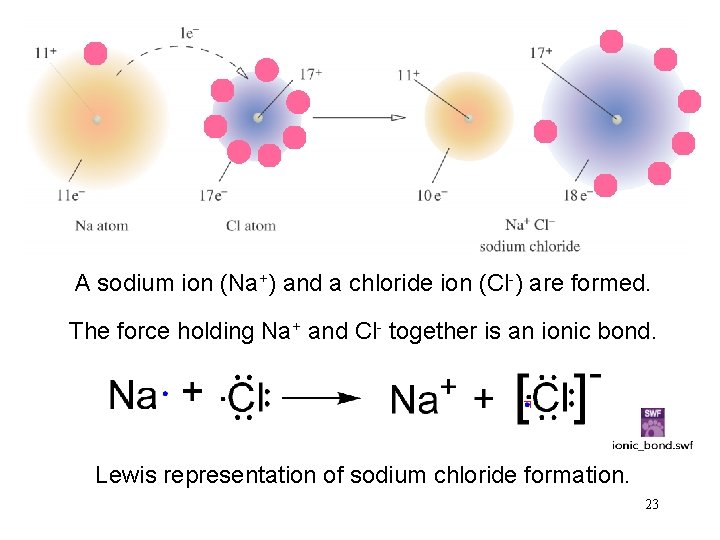
A sodium ion (Na+) and a chloride ion (Cl-) are formed. The 3 s electron of sodium transfers to the 3 p orbital of chlorine. The force holding Na+ and Cl- together is an ionic bond. Lewis representation of sodium chloride formation. 23
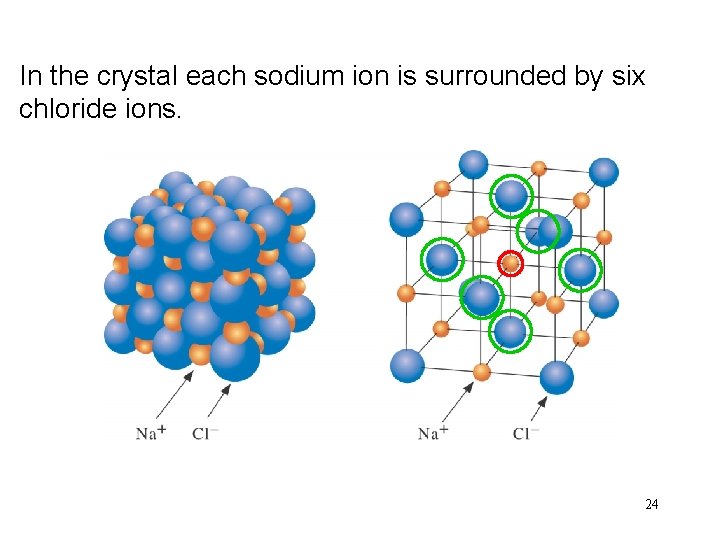
In Na. Cl the crystal is made each upsodium of cubicion crystals. is surrounded by six chloride ions. 24
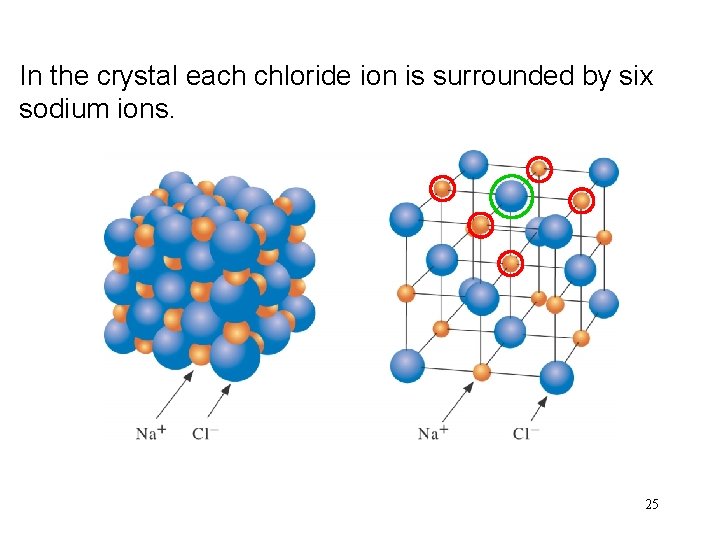
In the crystal each chloride ion is surrounded by six sodium ions. 25
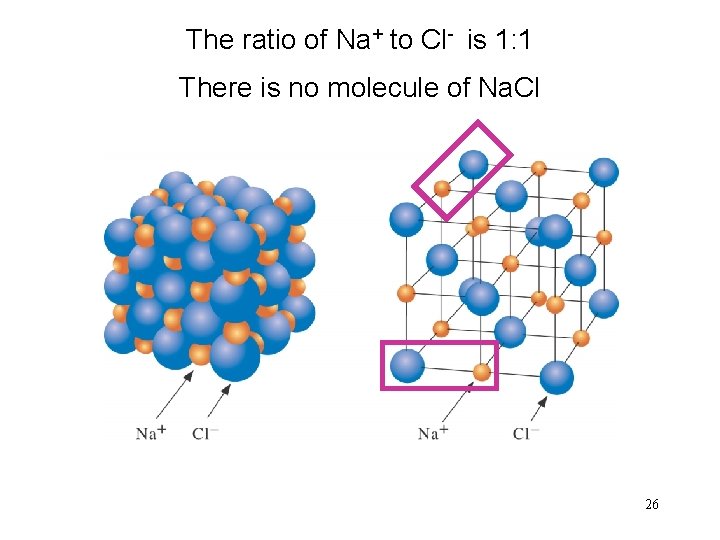
The ratio of Na+ to Cl- is 1: 1 There is no molecule of Na. Cl 26

The Covalent Bond Sharing Electrons 27

A covalent bond consists of a pair of electrons shared between two atoms. In the millions of chemical compounds that exist, the covalent bond is the predominant chemical bond. 28
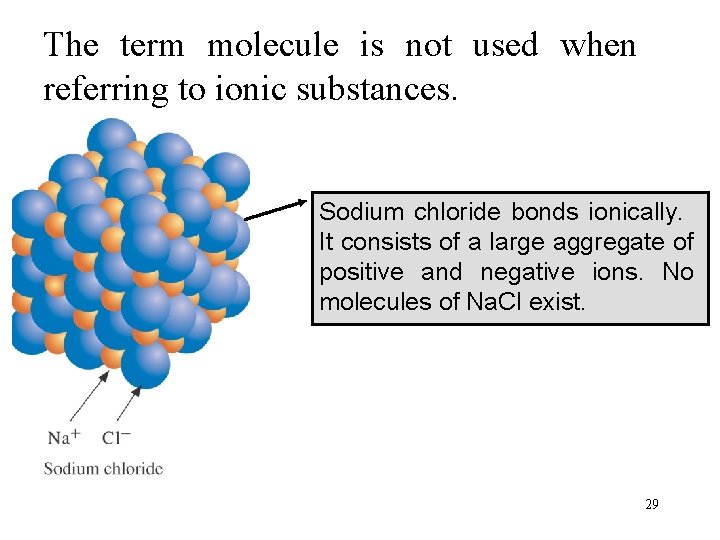
The term molecule is not used when referring to ionic substances. Sodium chloride bonds ionically. It consists of a large aggregate of positive and negative ions. No molecules of Na. Cl exist. 29
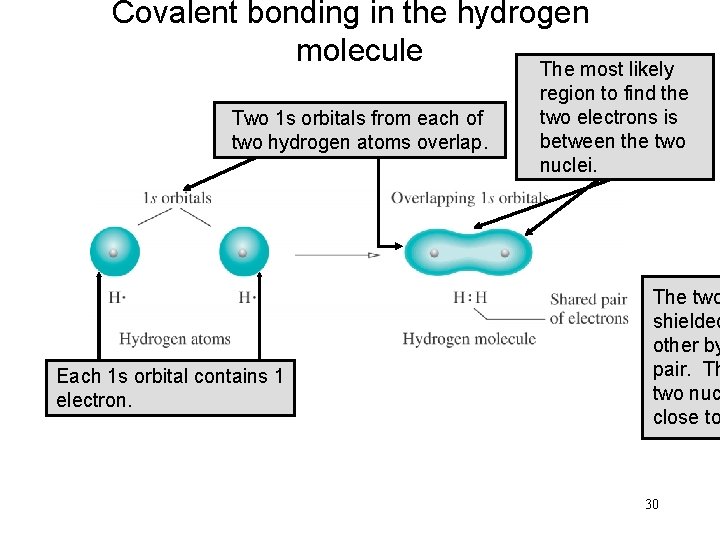
Covalent bonding in the hydrogen molecule The most likely Two 1 s 1 s orbitals from each of of two hydrogen atoms overlap. Each 1 s orbital contains 1 electron. The orbital of the region to find the electrons includes two electrons is both hydrogen between the two nuclei. The two shielded other by pair. Th two nuc close to 30
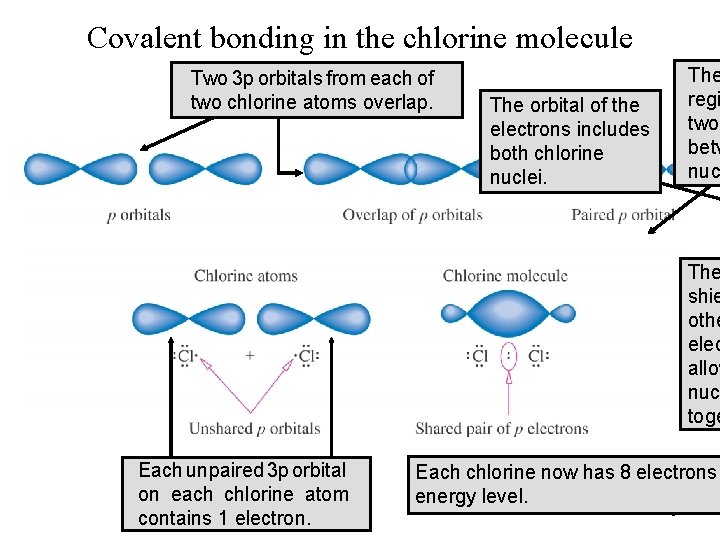
Covalent bonding in the chlorine molecule Two 3 p orbitalsfromeachofof two chlorine atoms overlap. The orbital of the electrons includes both chlorine nuclei. The regi two betw nuc The shie othe elec allow nuc toge Each unpaired 3 p orbital on each chlorine atom contains 1 electron. Each chlorine now has 8 electrons energy level. 31
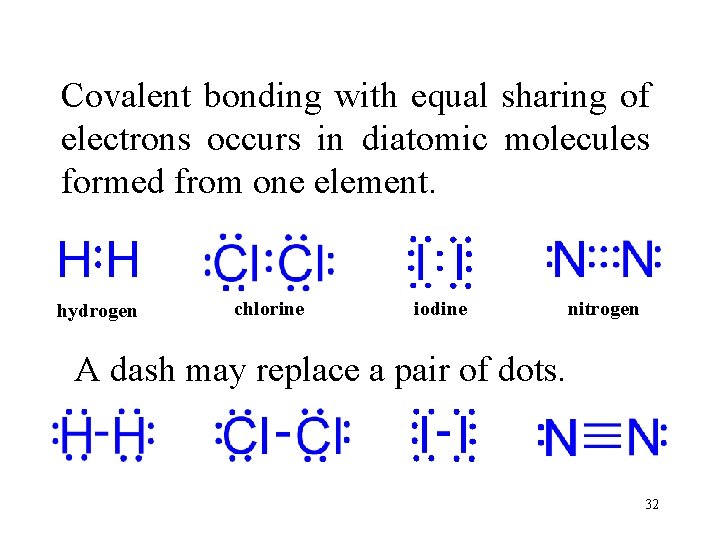
Covalent bonding with equal sharing of electrons occurs in diatomic molecules formed from one element. hydrogen chlorine iodine nitrogen A dash may replace a pair of dots. 32
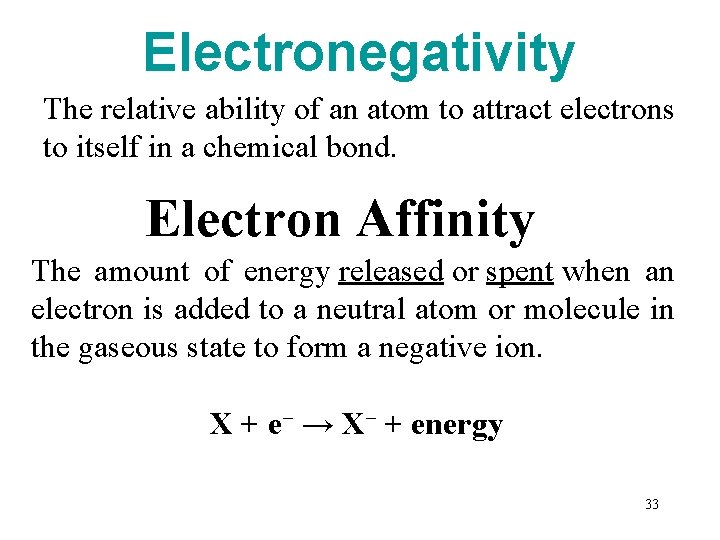
Electronegativity The relative ability of an atom to attract electrons to itself in a chemical bond. Electron Affinity The amount of energy released or spent when an electron is added to a neutral atom or molecule in the gaseous state to form a negative ion. X + e− → X− + energy 33

• If the two atoms that constitute a covalent bond are identical, then there is equal sharing of electrons. • This is called nonpolar covalent bonding. 34

• If the two atoms that constitute a covalent bond are not identical, then there is unequal sharing of electrons. • This is called polar covalent bonding. • One atom assumes a partial positive charge and the other atom assumes a partial negative charge. – This charge difference is a result of the unequal attractions the atoms have for their shared electron pair. 35
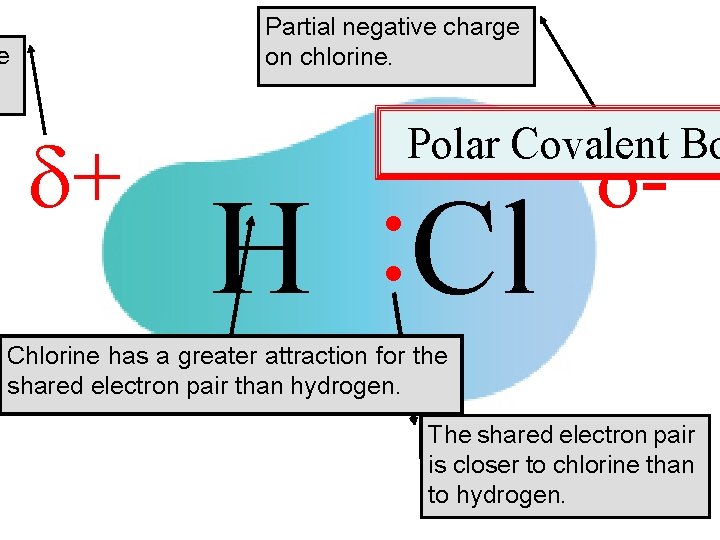
Partial negative charge on chlorine. e + : : H Cl - Polar Covalent Bo Chlorine has a greater attraction for the shared electron pair than hydrogen. Shared The shared electron pair is closer to chlorine than to hydrogen. 36
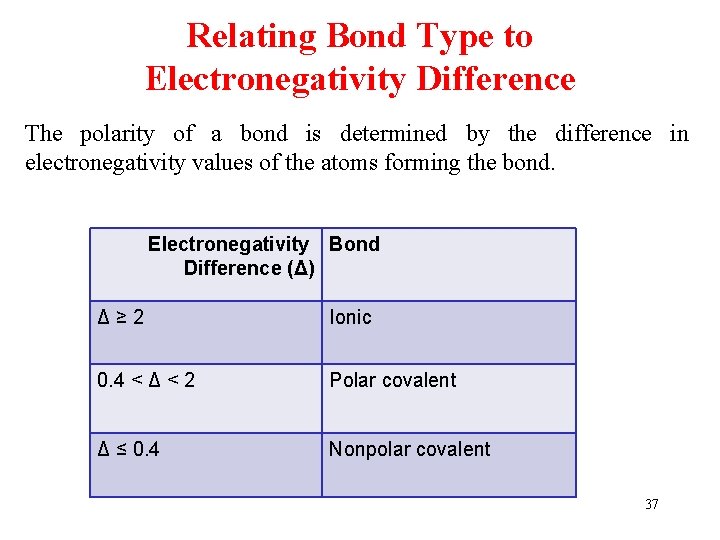
Relating Bond Type to Electronegativity Difference The polarity of a bond is determined by the difference in electronegativity values of the atoms forming the bond. Electronegativity Bond Difference (Δ) Δ≥ 2 Ionic 0. 4 ˂ Δ ˂ 2 Polar covalent Δ ≤ 0. 4 Nonpolar covalent 37
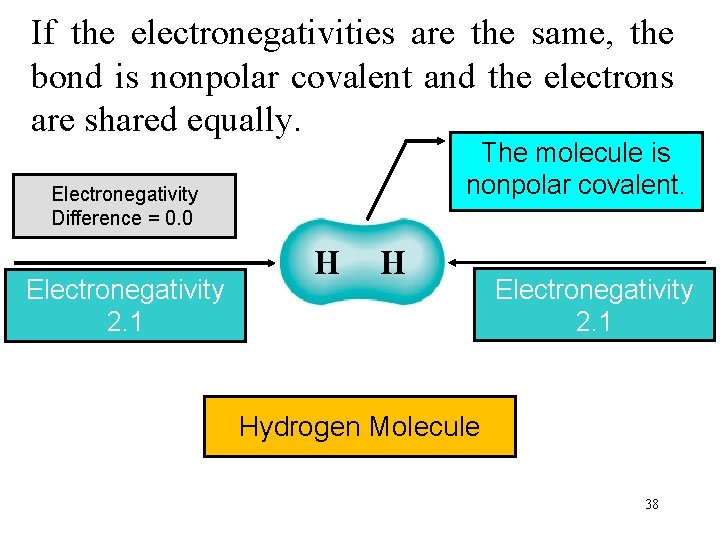
If the electronegativities are the same, the bond is nonpolar covalent and the electrons are shared equally. The molecule is nonpolar covalent. Electronegativity Difference = 0. 0 Electronegativity 2. 1 H H Electronegativity 2. 1 Hydrogen Molecule 38
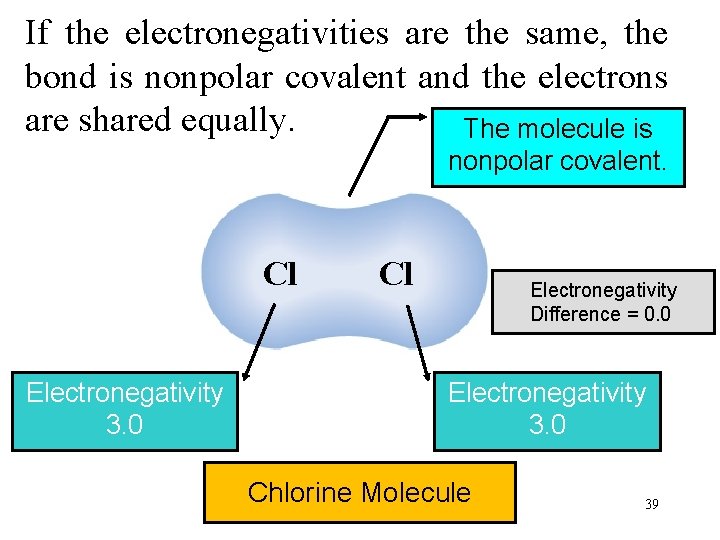
If the electronegativities are the same, the bond is nonpolar covalent and the electrons are shared equally. The molecule is nonpolar covalent. Cl Electronegativity 3. 0 Cl Electronegativity Difference = 0. 0 Electronegativity 3. 0 Chlorine Molecule 39
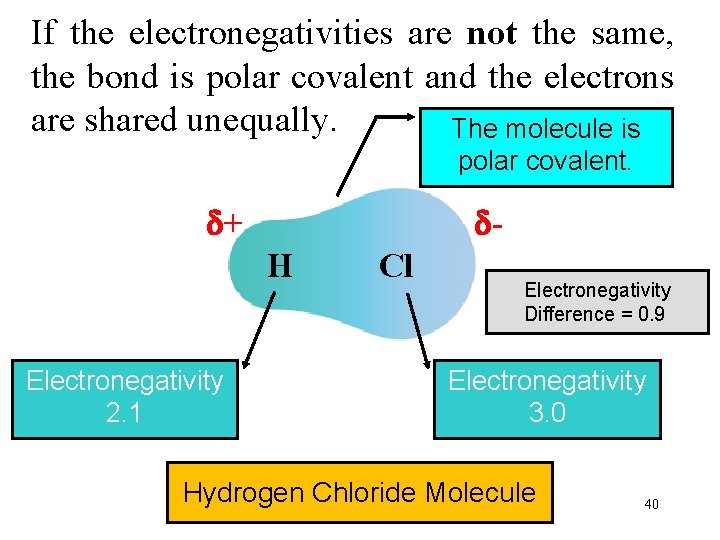
If the electronegativities are not the same, the bond is polar covalent and the electrons are shared unequally. The molecule is polar covalent. + H Electronegativity 2. 1 Cl Electronegativity Difference = 0. 9 Electronegativity 3. 0 Hydrogen Chloride Molecule 40
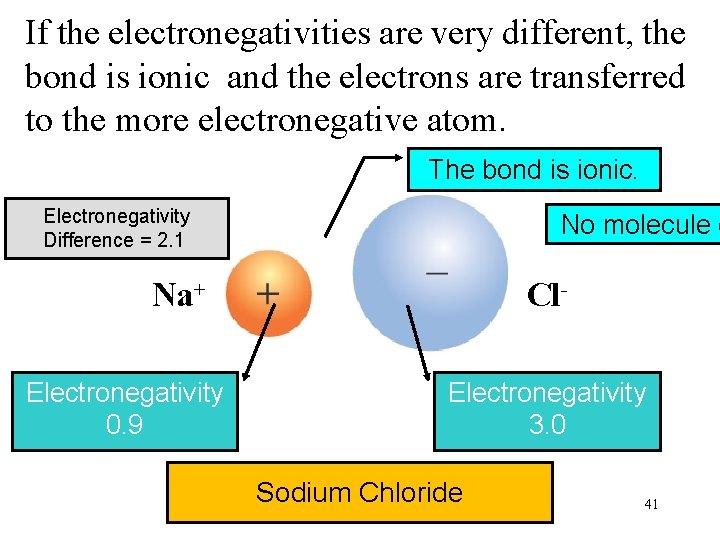
If the electronegativities are very different, the bond is ionic and the electrons are transferred to the more electronegative atom. The bond is ionic. Electronegativity Difference = 2. 1 No molecule e Na+ Electronegativity 0. 9 Cl. Electronegativity 3. 0 Sodium Chloride 41
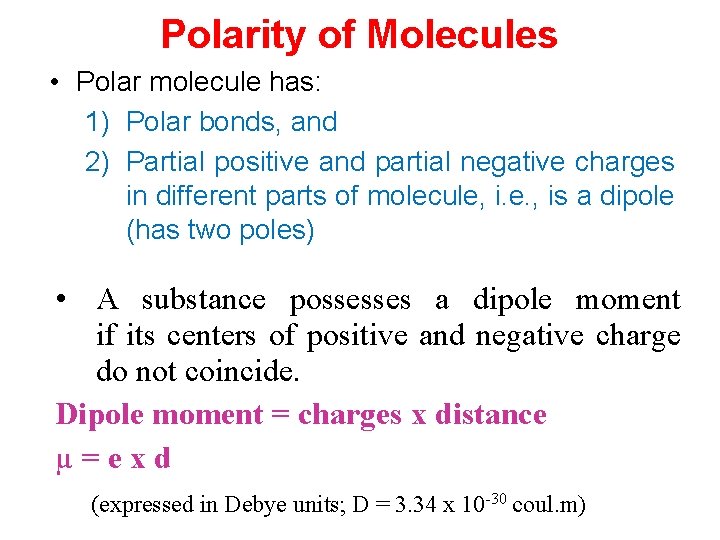
Polarity of Molecules • Polar molecule has: 1) Polar bonds, and 2) Partial positive and partial negative charges in different parts of molecule, i. e. , is a dipole (has two poles) • A substance possesses a dipole moment if its centers of positive and negative charge do not coincide. Dipole moment = charges x distance µ=exd (expressed in Debye units; D = 3. 34 x 10 -30 coul. m)
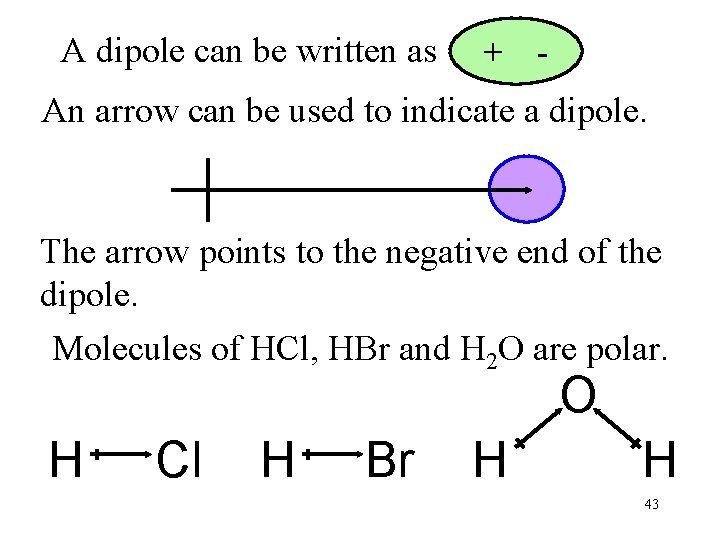
A dipole can be written as + - An arrow can be used to indicate a dipole. The arrow points to the negative end of the dipole. Molecules of HCl, HBr and H 2 O are polar. O H Cl H Br H H 43
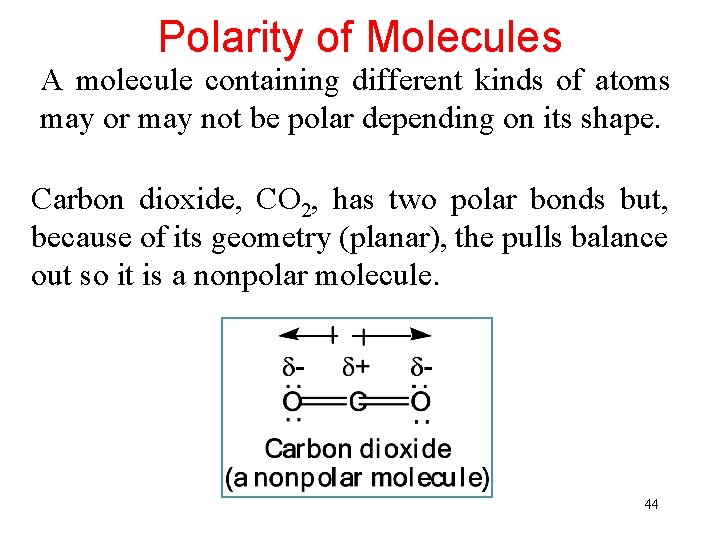
Polarity of Molecules A molecule containing different kinds of atoms may or may not be polar depending on its shape. Carbon dioxide, CO 2, has two polar bonds but, because of its geometry (planar), the pulls balance out so it is a nonpolar molecule. 44
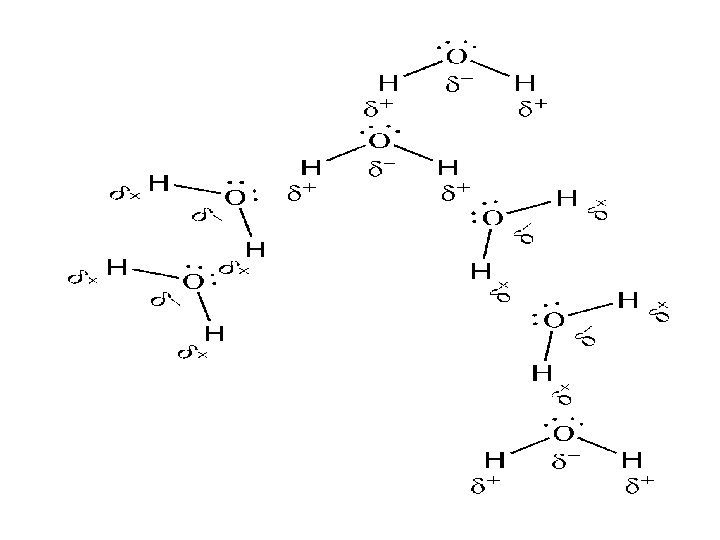
Hydrogen Bonding Ø Occurs when H is involved in a strongly polar bond with O, N or F. Ø The H nucleus (just a proton) is attracted to the lone pairs of these highly electronegative atoms. Ø The result is a network of strong intermolecular interactions between H atoms and available lone pairs. Ø Occurs in H 2 O, NH 3 and HF. • The primary reason H 2 O doesn’t behave as almost all other substances in the known universe. • H 2 O expands when it freezes everything else contracts
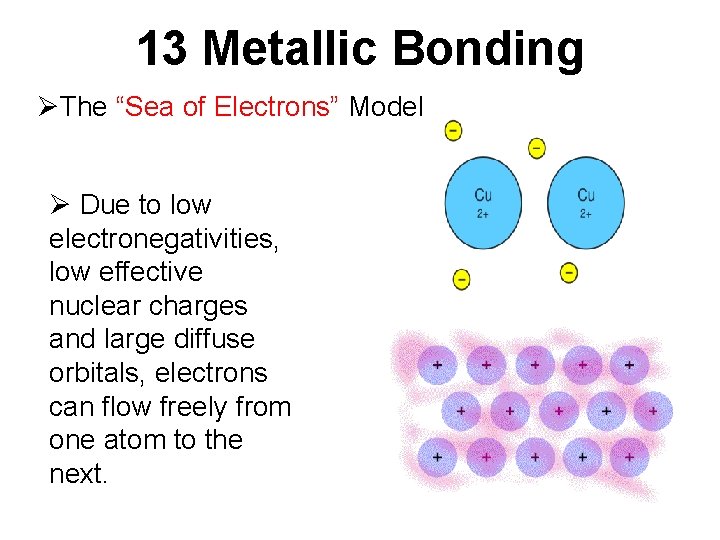
13 Metallic Bonding ØThe “Sea of Electrons” Model Ø Due to low electronegativities, low effective nuclear charges and large diffuse orbitals, electrons can flow freely from one atom to the next.

48
- Slides: 48