6 5 Covalent Compounds and Their names Covalent








- Slides: 22

6. 5 Covalent Compounds and Their names Covalent compounds= nonmetal + nonmetal, OR metalloid + nonmetal Electrons are SHARED to fulfill The octet rule 1 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

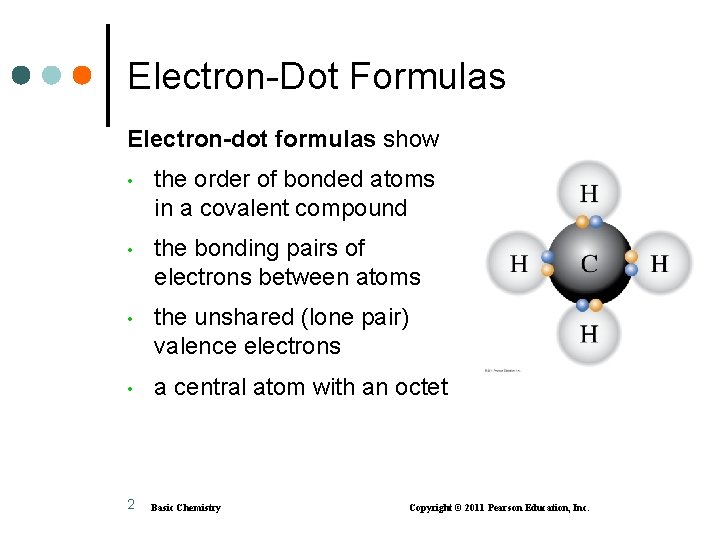
Electron-Dot Formulas Electron-dot formulas show • the order of bonded atoms in a covalent compound • the bonding pairs of electrons between atoms • the unshared (lone pair) valence electrons • a central atom with an octet 2 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

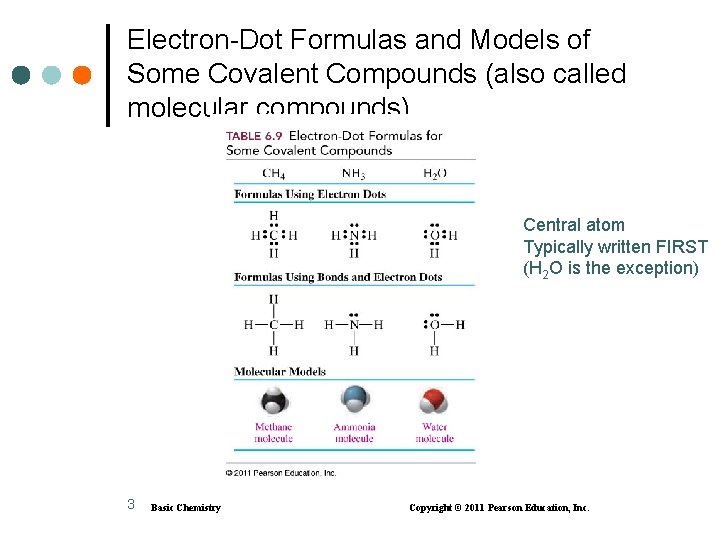
Electron-Dot Formulas and Models of Some Covalent Compounds (also called molecular compounds) Central atom Typically written FIRST (H 2 O is the exception) 3 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

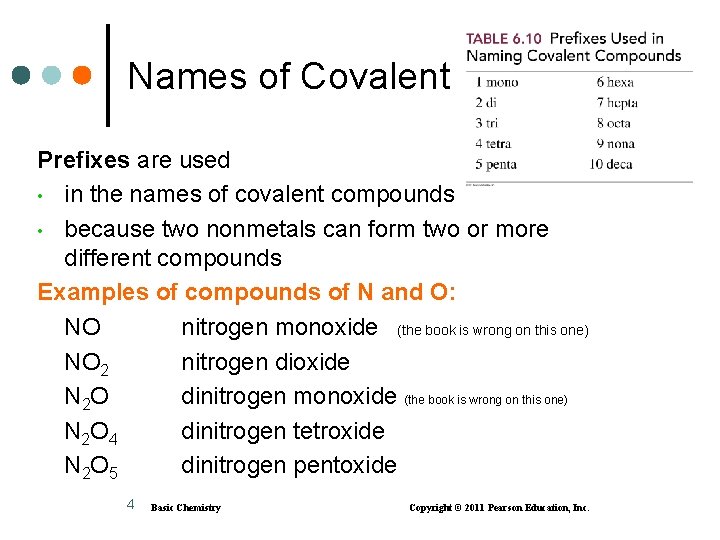
Names of Covalent Compounds Prefixes are used • in the names of covalent compounds • because two nonmetals can form two or more different compounds Examples of compounds of N and O: NO nitrogen monoxide (the book is wrong on this one) NO 2 nitrogen dioxide N 2 O dinitrogen monoxide (the book is wrong on this one) N 2 O 4 dinitrogen tetroxide N 2 O 5 dinitrogen pentoxide 4 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Guide to Naming Covalent Compounds 5 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

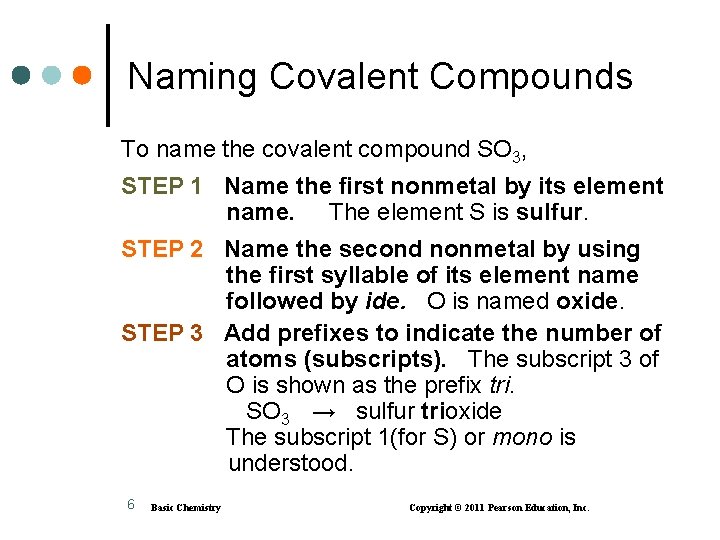
Naming Covalent Compounds To name the covalent compound SO 3, STEP 1 Name the first nonmetal by its element name. The element S is sulfur. STEP 2 Name the second nonmetal by using the first syllable of its element name followed by ide. O is named oxide. STEP 3 Add prefixes to indicate the number of atoms (subscripts). The subscript 3 of O is shown as the prefix tri. SO 3 → sulfur trioxide The subscript 1(for S) or mono is understood. 6 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

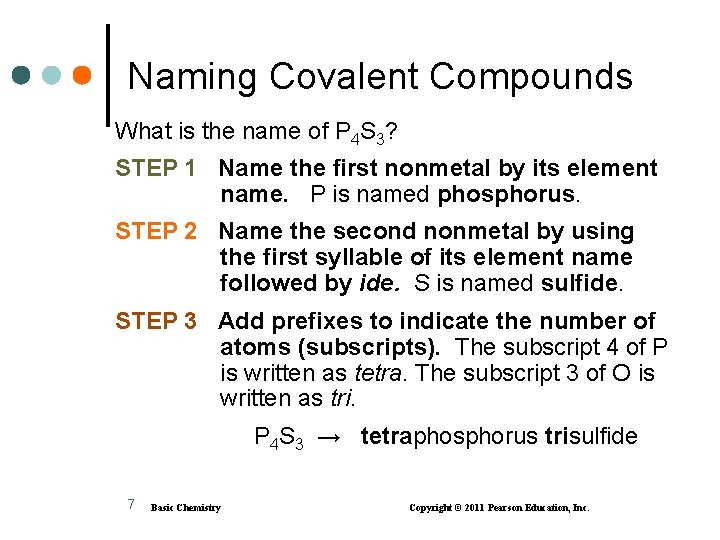
Naming Covalent Compounds What is the name of P 4 S 3? STEP 1 Name the first nonmetal by its element name. P is named phosphorus. STEP 2 Name the second nonmetal by using the first syllable of its element name followed by ide. S is named sulfide. STEP 3 Add prefixes to indicate the number of atoms (subscripts). The subscript 4 of P is written as tetra. The subscript 3 of O is written as tri. P 4 S 3 → tetraphosphorus trisulfide 7 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Learning Check Select the correct name for each compound. A. Si. Cl 4 1) silicon chloride 2) tetrasilicon chloride 3) silicon tetrachloride B. P 2 O 5 1) phosphorus oxide 2) phosphorus pentoxide 3) diphosphorus pentoxide C. Cl 2 O 7 1) dichlorine heptoxide 2) dichlorine oxide 3) chlorine heptoxide 8 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Select the correct name for each compound. A. B. C. 9 Si. Cl 4 P 2 O 5 Cl 2 O 7 3) silicon tetrachloride 3) diphosphorus pentoxide 1) dichlorine heptoxide Basic Chemistry Copyright © 2011 Pearson Education, Inc.

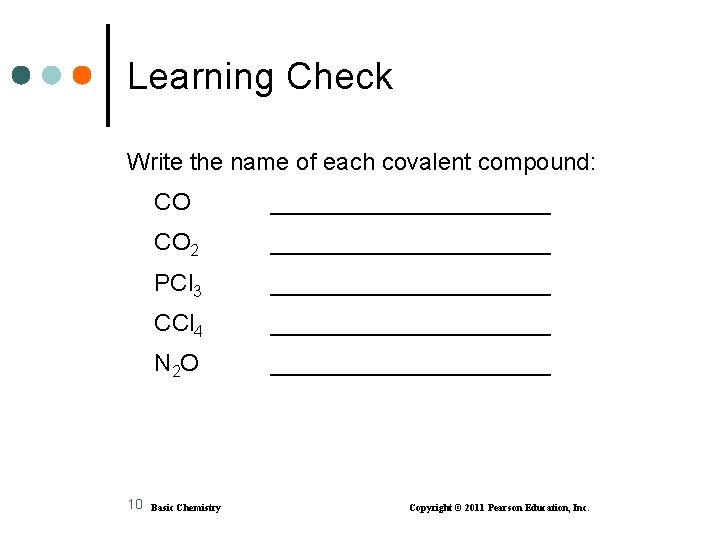
Learning Check Write the name of each covalent compound: 10 CO ___________ CO 2 ___________ PCl 3 ___________ CCl 4 ___________ N 2 O ___________ Basic Chemistry Copyright © 2011 Pearson Education, Inc.

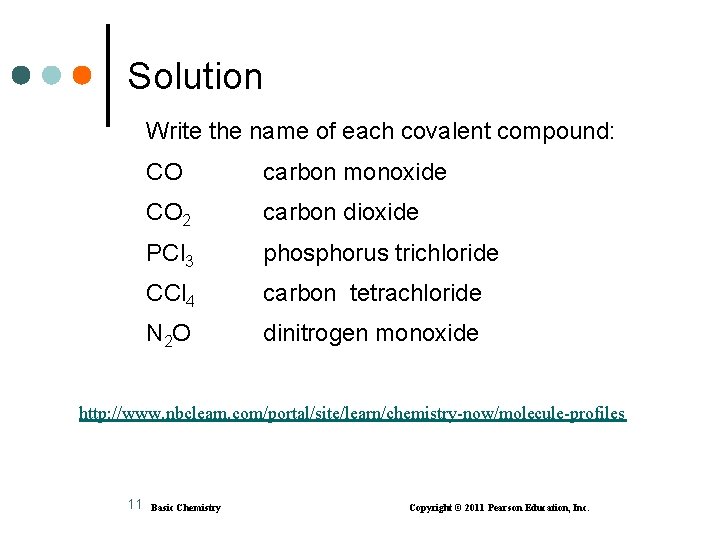
Solution Write the name of each covalent compound: CO carbon monoxide CO 2 carbon dioxide PCl 3 phosphorus trichloride CCl 4 carbon tetrachloride N 2 O dinitrogen monoxide http: //www. nbclearn. com/portal/site/learn/chemistry-now/molecule-profiles 11 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Guide to Writing Formulas Example: Write the formula for carbon disulfide. STEP 1 Write the symbols in the order of the elements in the name. C S STEP 2 Write any prefixes as subscripts. No prefix for carbon means one(1) C. The prefix di for sulfur means two(2) S. formula: CS 2 12 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

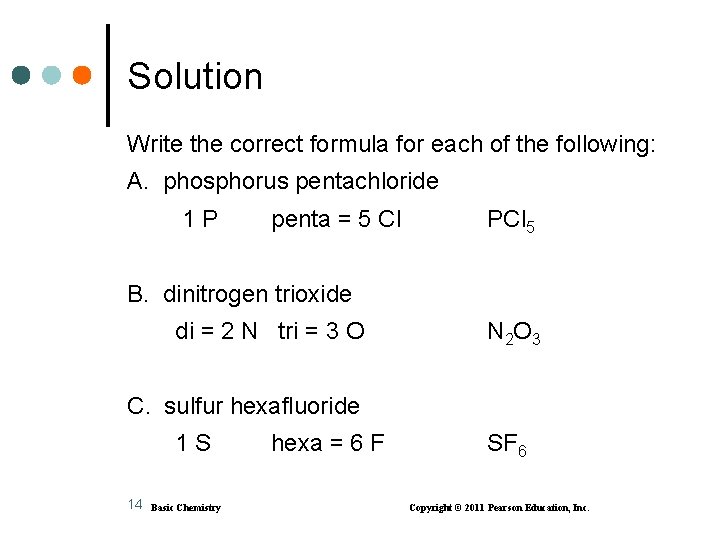
Learning Check Write the correct formula for each of the following: A. phosphorus pentachloride B. dinitrogen trioxide C. sulfur hexafluoride 13 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Write the correct formula for each of the following: A. phosphorus pentachloride 1 P penta = 5 Cl PCl 5 B. dinitrogen trioxide di = 2 N tri = 3 O N 2 O 3 C. sulfur hexafluoride 1 S 14 hexa = 6 F SF 6 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Learning Check Identify each compound as ionic or covalent and give its correct name. A. SO 3 B. Mn. Cl 2 C. (NH 4)3 PO 4 D. Cu 2 CO 3 E. N 2 O 4 15 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution Identify each compound as ionic or covalent and give its correct name. A. SO 3 covalent; sulfur trioxide B. Mn. Cl 2 ionic; manganese(II) chloride C. (NH 4)3 PO 3 ionic; ammonium phosphite D. Cu 2 CO 3 ionic; copper(I) carbonate E. N 2 O 4 covalent; dinitrogen tetroxide 16 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

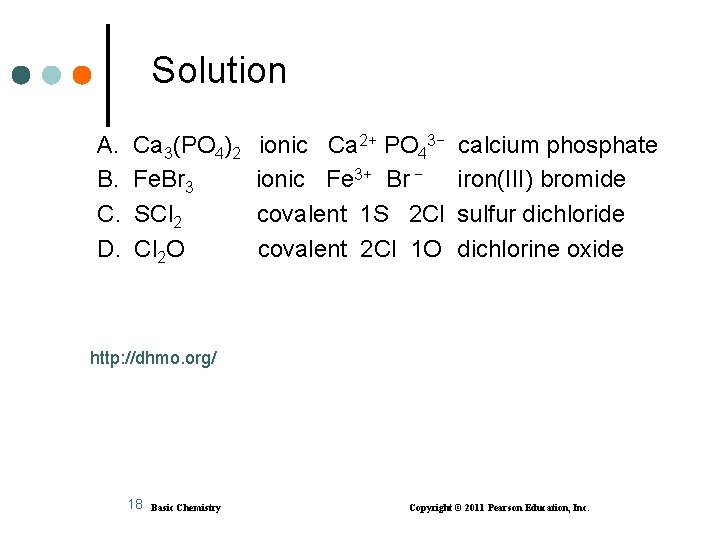
Learning Check Identify each compound as ionic or covalent and give its correct name. A. Ca 3(PO 4)2 B. Fe. Br 3 C. SCl 2 D. Cl 2 O 17 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Solution A. B. C. D. Ca 3(PO 4)2 Fe. Br 3 SCl 2 O ionic Ca 2+ PO 43− ionic Fe 3+ Br − covalent 1 S 2 Cl covalent 2 Cl 1 O calcium phosphate iron(III) bromide sulfur dichloride dichlorine oxide http: //dhmo. org/ 18 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

H 2, A Covalent Molecule In a hydrogen (H 2) molecule, • two hydrogen atoms share electrons to form a covalent single bond • each H atom acquires two (2) electrons • each H becomes stable like helium (He) 19 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

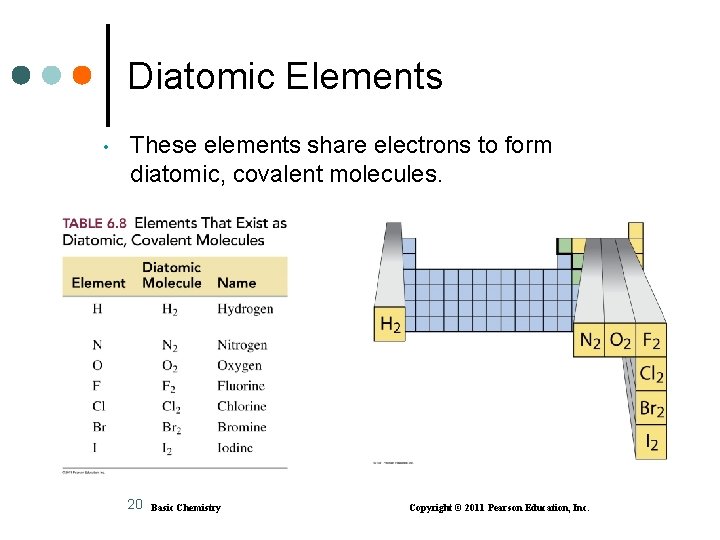
Diatomic Elements • These elements share electrons to form diatomic, covalent molecules. 20 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

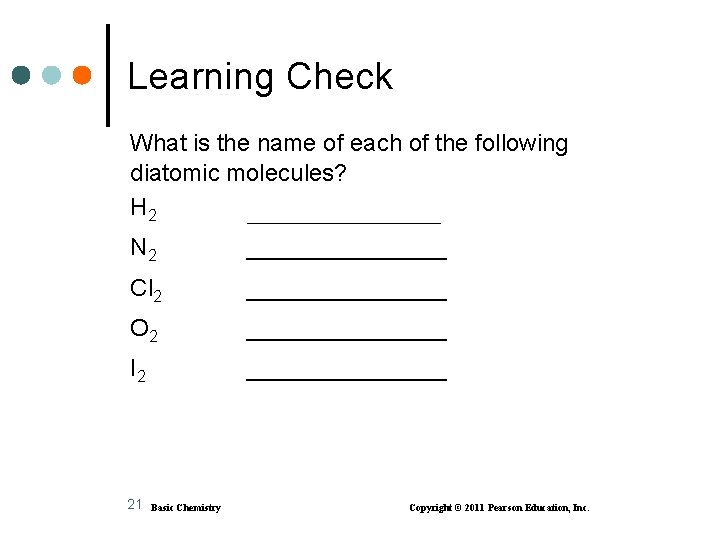
Learning Check What is the name of each of the following diatomic molecules? H 2 ___________ N 2 ________ Cl 2 ________ O 2 ________ I 2 ________ 21 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

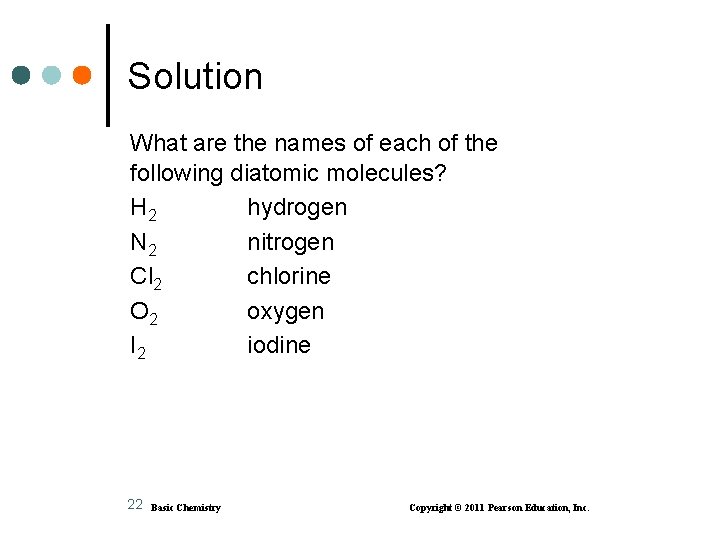
Solution What are the names of each of the following diatomic molecules? H 2 hydrogen N 2 nitrogen Cl 2 chlorine O 2 oxygen I 2 iodine 22 Basic Chemistry Copyright © 2011 Pearson Education, Inc.