6 2 Factors Affecting Reaction Rate Many factors



- Slides: 6

6. 2 – Factors Affecting Reaction Rate • Many factors can determine the rate a chemical reaction occurs. • Temperature • Concentration • Surface area A bicycle chain slowly rusts • Catalysts See pages 272 - 273

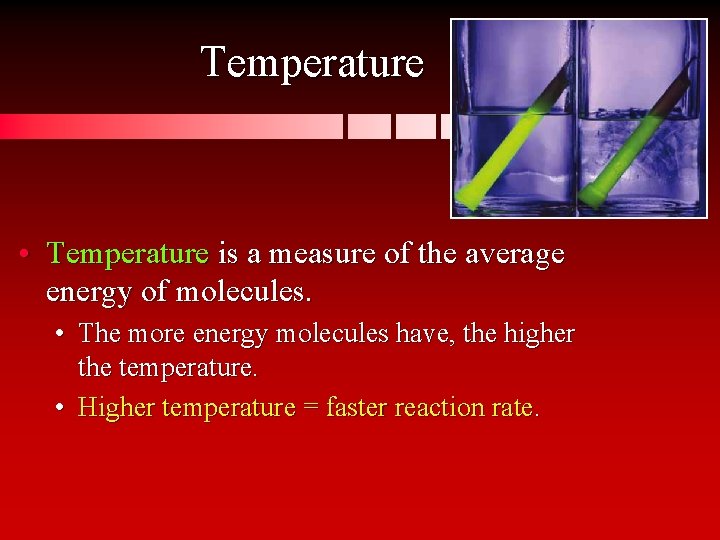
Temperature • Temperature is a measure of the average energy of molecules. • The more energy molecules have, the higher the temperature. • Higher temperature = faster reaction rate.

Concentration Changing the concentration of O 2 changes the intensity of flames. • Concentration refers to how many molecules of a substance exist in a certain volume (g/L). • Higher Concentration = faster reaction rate - Since there are molecules per unit volume in high concentrations, there are more opportunities for molecules to collide & react.

Surface Area • Surface area is how much of the material is exposed to the air or other reactants. • Higher surface area = faster reaction rate. • Since there are more atoms and compounds exposed to react, more reactions take place. Steel wool (on the right) is made up of small strands of steel, and therefore has much more surface area than an equivalent amount of solid steel.

Catalysts • A catalyst is a chemical that allows a reaction to occur more quickly without actually participating in the reaction itself. • Catalyst = faster reaction rate (not part of the reactants). • Catalysts lower the amount of energy needed to break the bonds in the reactants. • Eg. Enzymes are biological catalysts Salivary amylase increases the digestion of starches.

Catalytic Converters • A catalytic converter is a device installed in all cars to decrease pollution. • Catalysts found in the honeycomb-shaped filters in the converter help to change many of the pollutants. • Poisonous CO is changed into CO 2 • Hydrocarbons are converted into CO 2 & H 2 O • Nitrogen oxides are changed into N 2 & O 2 • 2 N 2 O 3 2 N 2 + 3 O 2 Take the Section 6. 2 Quiz