6 2 Empirical and Molecular Formulas Types of





- Slides: 22

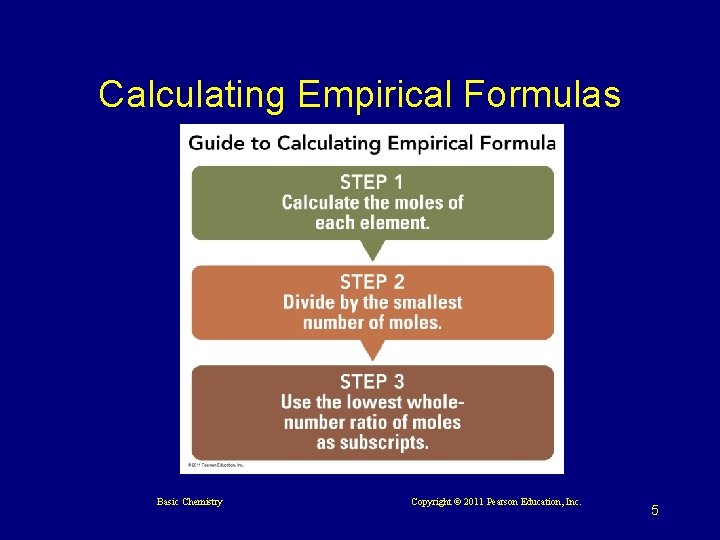
6. 2 Empirical and Molecular Formulas

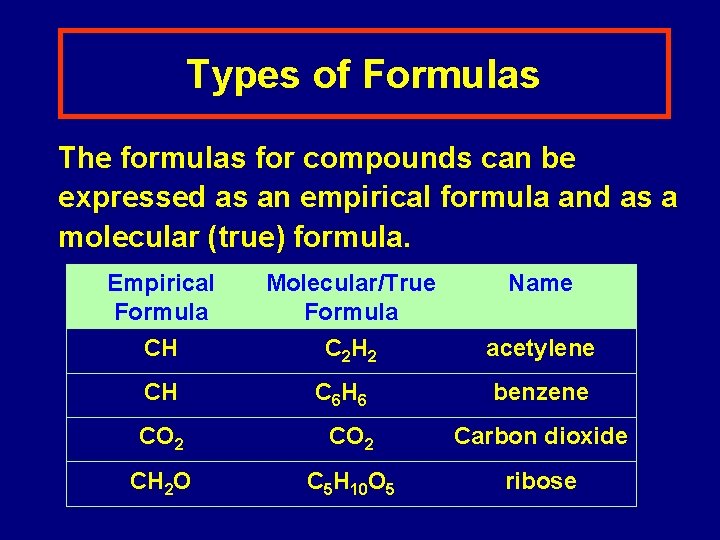
Types of Formulas The formulas for compounds can be expressed as an empirical formula and as a molecular (true) formula. Empirical Formula Molecular/True Formula Name CH C 2 H 2 acetylene CH C 6 H 6 benzene CO 2 Carbon dioxide CH 2 O C 5 H 10 O 5 ribose

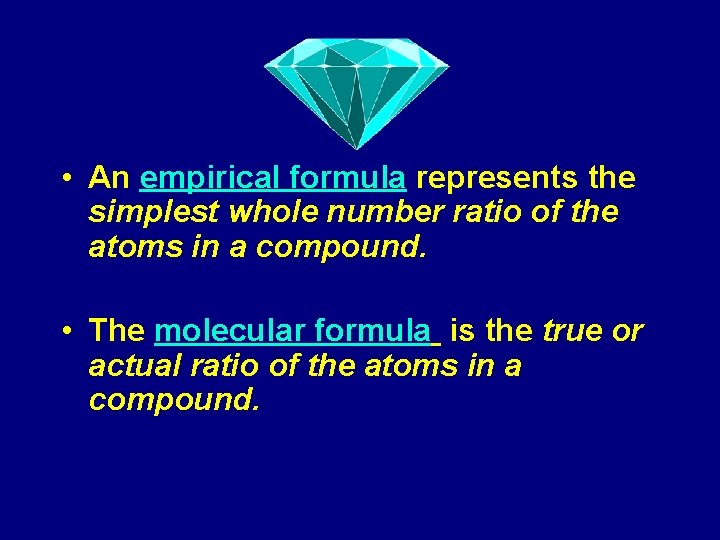
• An empirical formula represents the simplest whole number ratio of the atoms in a compound. • The molecular formula is the true or actual ratio of the atoms in a compound.

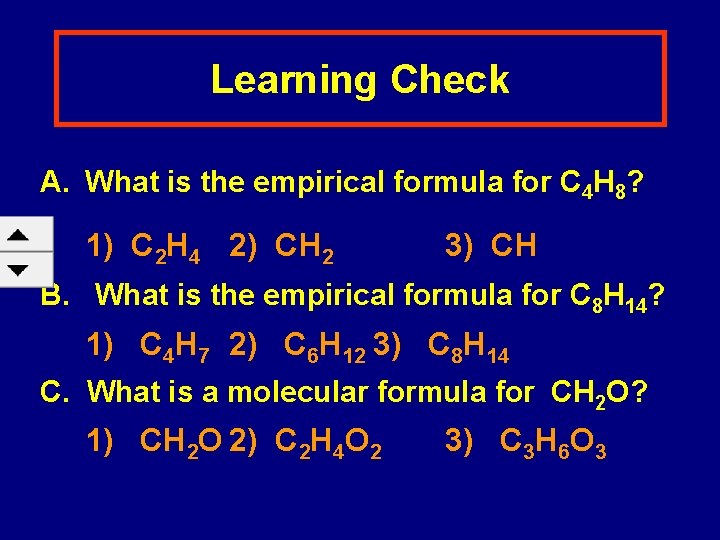
Learning Check A. What is the empirical formula for C 4 H 8? 1) C 2 H 4 2) CH 2 3) CH B. What is the empirical formula for C 8 H 14? 1) C 4 H 7 2) C 6 H 12 3) C 8 H 14 C. What is a molecular formula for CH 2 O? 1) CH 2 O 2) C 2 H 4 O 2 3) C 3 H 6 O 3

Calculating Empirical Formulas Basic Chemistry Copyright © 2011 Pearson Education, Inc. 5

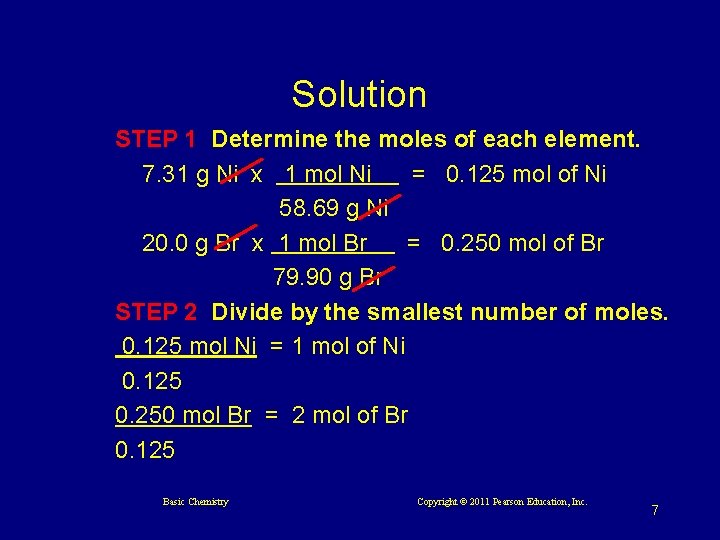
Learning Check A compound contains 7. 31 g Ni and 20. 0 g Br. Calculate its empirical (simplest) formula. Basic Chemistry Copyright © 2011 Pearson Education, Inc. 6

Solution STEP 1 Determine the moles of each element. 7. 31 g Ni x 1 mol Ni = 0. 125 mol of Ni 58. 69 g Ni 20. 0 g Br x 1 mol Br = 0. 250 mol of Br 79. 90 g Br STEP 2 Divide by the smallest number of moles. 0. 125 mol Ni = 1 mol of Ni 0. 125 0. 250 mol Br = 2 mol of Br 0. 125 Basic Chemistry Copyright © 2011 Pearson Education, Inc. 7

Solution (continued) STEP 3 Use the lowest whole-number ratio of moles as subscripts. Ni. Br 2 Basic Chemistry Copyright © 2011 Pearson Education, Inc. 8

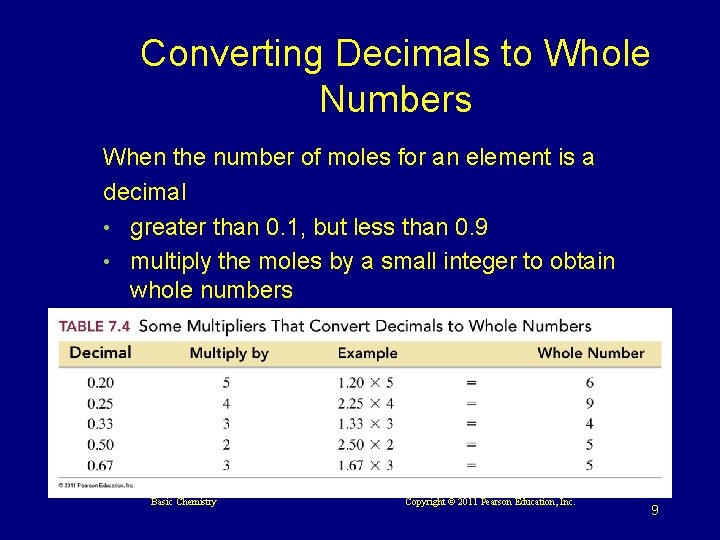
Converting Decimals to Whole Numbers When the number of moles for an element is a decimal • greater than 0. 1, but less than 0. 9 • multiply the moles by a small integer to obtain whole numbers Basic Chemistry Copyright © 2011 Pearson Education, Inc. 9

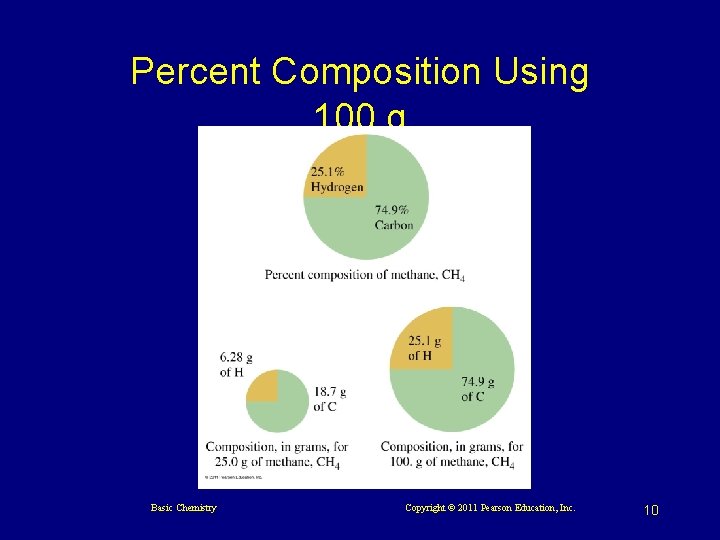
Percent Composition Using 100 g Basic Chemistry Copyright © 2011 Pearson Education, Inc. 10

Finding the Empirical Formula “Percent to mass Mass to mole Divide by small Multiply ‘til whole”

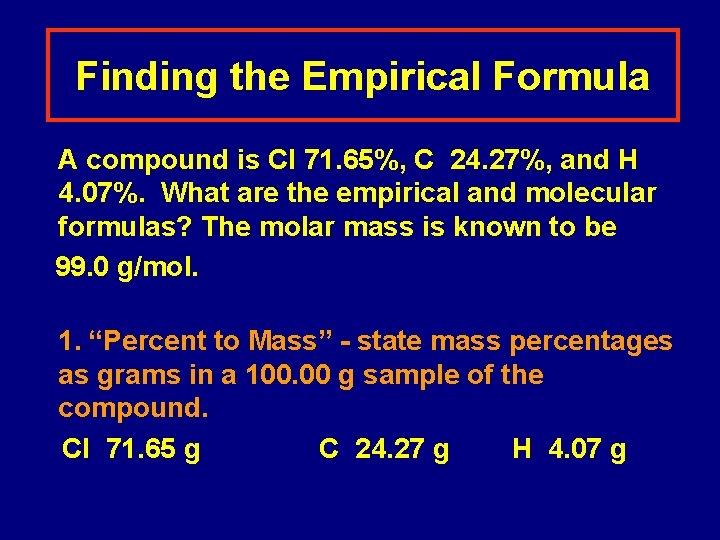
Finding the Empirical Formula A compound is Cl 71. 65%, C 24. 27%, and H 4. 07%. What are the empirical and molecular formulas? The molar mass is known to be 99. 0 g/mol. 1. “Percent to Mass” - state mass percentages as grams in a 100. 00 g sample of the compound. Cl 71. 65 g C 24. 27 g H 4. 07 g

2. “Mass to Moles” 71. 65 g Cl x 1 mol Cl = 2. 02 mol Cl 35. 5 g Cl 24. 27 g C x 1 mol C 12. 0 g C = 2. 02 mol C 4. 07 g H x 1 mol H 1. 01 g H = 4. 04 mol H

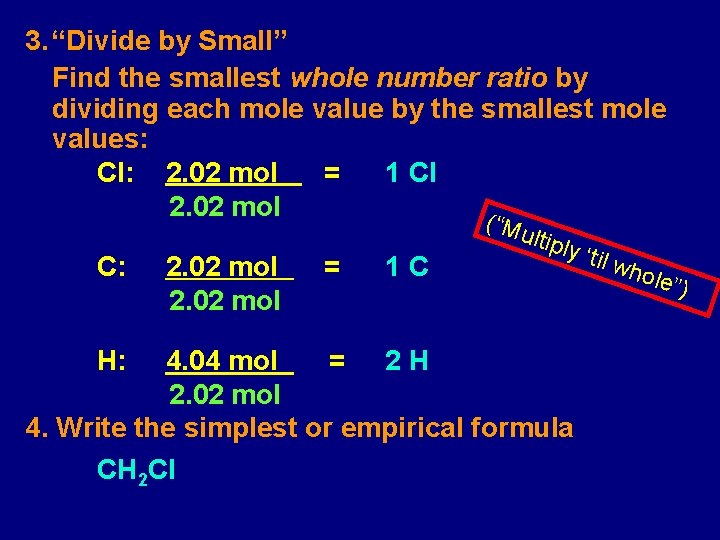
3. “Divide by Small” Find the smallest whole number ratio by dividing each mole value by the smallest mole values: Cl: 2. 02 mol = 1 Cl 2. 02 mol ( “Mu C: H: 2. 02 mol = 1 C ltiply 4. 04 mol = 2 H 2. 02 mol 4. Write the simplest or empirical formula CH 2 Cl ‘til w hole ”)

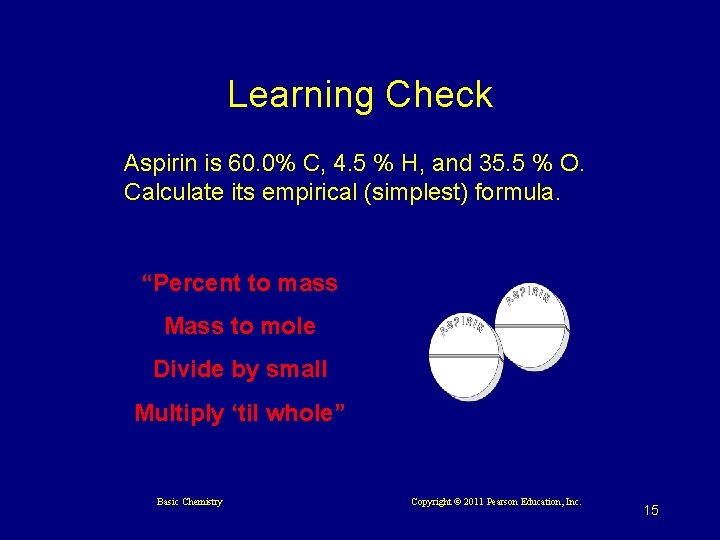
Learning Check Aspirin is 60. 0% C, 4. 5 % H, and 35. 5 % O. Calculate its empirical (simplest) formula. “Percent to mass Mass to mole Divide by small Multiply ‘til whole” Basic Chemistry Copyright © 2011 Pearson Education, Inc. 15

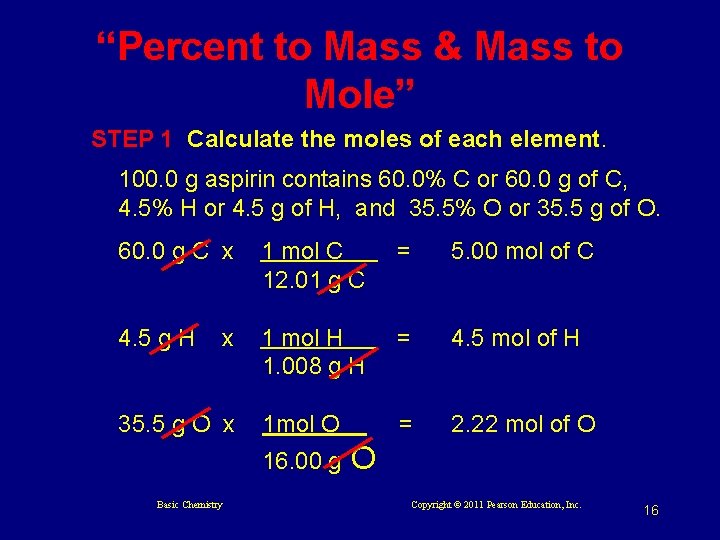
“Percent to Mass & Mass to Mole” STEP 1 Calculate the moles of each element. 100. 0 g aspirin contains 60. 0% C or 60. 0 g of C, 4. 5% H or 4. 5 g of H, and 35. 5% O or 35. 5 g of O. 60. 0 g C x 1 mol C 12. 01 g C = 5. 00 mol of C 4. 5 g H 1 mol H 1. 008 g H = 4. 5 mol of H 1 mol O = 2. 22 mol of O x 35. 5 g O x 16. 00 g Basic Chemistry O Copyright © 2011 Pearson Education, Inc. 16

“Divide by Small” STEP 2 Divide by the smallest number of moles. 5. 00 mol C 2. 22 4. 5 mol H 2. 22 mol O 2. 22 Basic Chemistry = 2. 25 mol of C = 2. 0 mol of H = 1. 00 mol of O Copyright © 2011 Pearson Education, Inc. 17

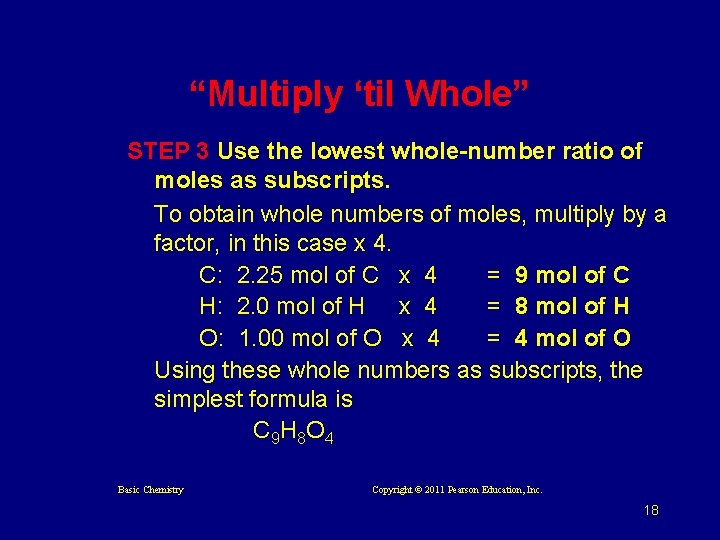
“Multiply ‘til Whole” STEP 3 Use the lowest whole-number ratio of moles as subscripts. To obtain whole numbers of moles, multiply by a factor, in this case x 4. C: 2. 25 mol of C x 4 = 9 mol of C H: 2. 0 mol of H x 4 = 8 mol of H O: 1. 00 mol of O x 4 = 4 mol of O Using these whole numbers as subscripts, the simplest formula is C 9 H 8 O 4 Basic Chemistry Copyright © 2011 Pearson Education, Inc. 18

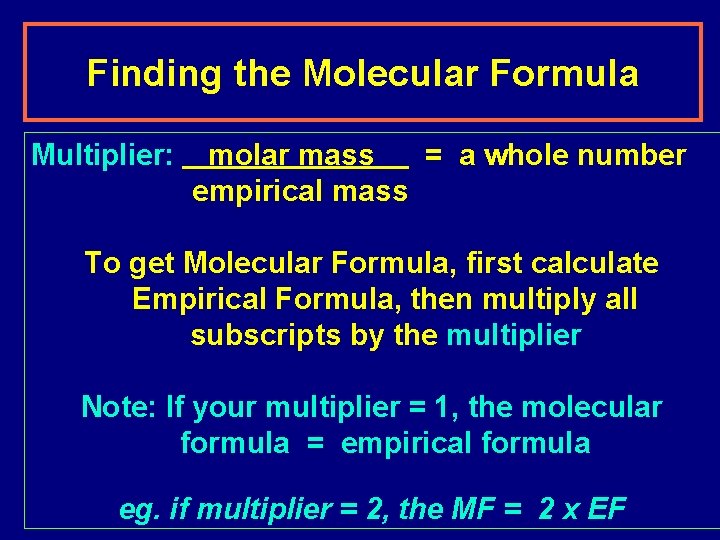
Finding the Molecular Formula Multiplier: molar mass = a whole number empirical mass To get Molecular Formula, first calculate Empirical Formula, then multiply all subscripts by the multiplier Note: If your multiplier = 1, the molecular formula = empirical formula eg. if multiplier = 2, the MF = 2 x EF

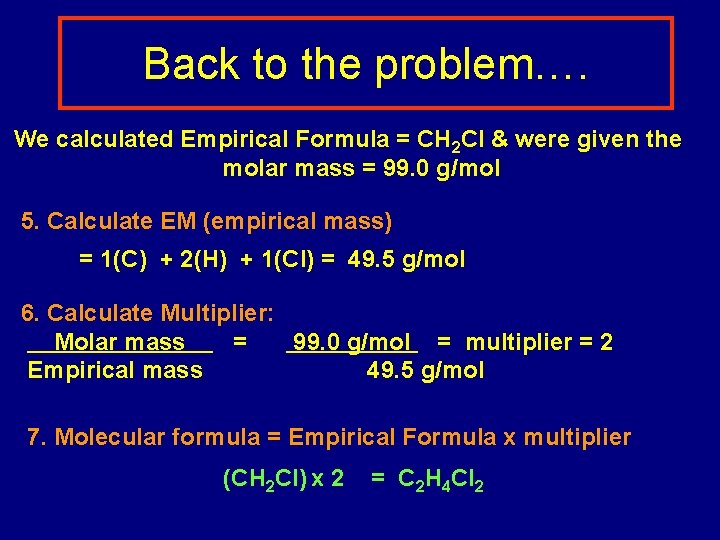
Back to the problem…. We calculated Empirical Formula = CH 2 Cl & were given the molar mass = 99. 0 g/mol 5. Calculate EM (empirical mass) = 1(C) + 2(H) + 1(Cl) = 49. 5 g/mol 6. Calculate Multiplier: Molar mass = 99. 0 g/mol = multiplier = 2 Empirical mass 49. 5 g/mol 7. Molecular formula = Empirical Formula x multiplier (CH 2 Cl) x 2 = C 2 H 4 Cl 2

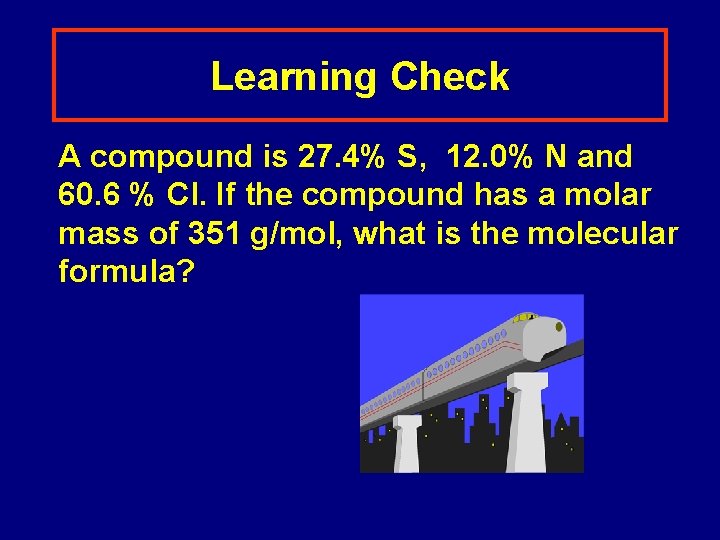
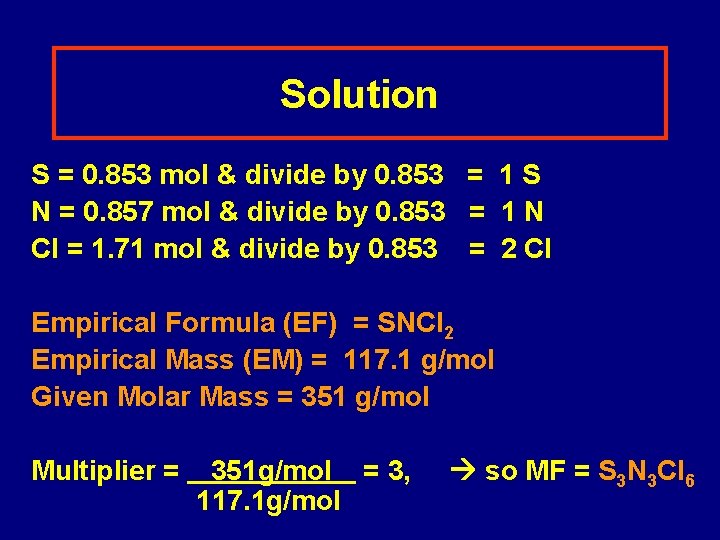
Learning Check A compound is 27. 4% S, 12. 0% N and 60. 6 % Cl. If the compound has a molar mass of 351 g/mol, what is the molecular formula?

Solution S = 0. 853 mol & divide by 0. 853 = 1 S N = 0. 857 mol & divide by 0. 853 = 1 N Cl = 1. 71 mol & divide by 0. 853 = 2 Cl Empirical Formula (EF) = SNCl 2 Empirical Mass (EM) = 117. 1 g/mol Given Molar Mass = 351 g/mol Multiplier = 351 g/mol = 3, 117. 1 g/mol so MF = S 3 N 3 Cl 6