6 1 Vocabulary SolventThe substance that is more

6. 1 Vocabulary
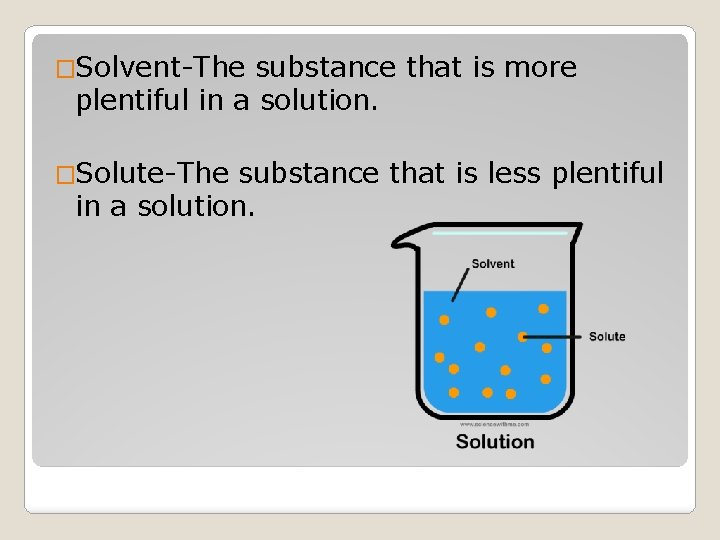
�Solvent-The substance that is more plentiful in a solution. �Solute-The substance that is less plentiful in a solution.
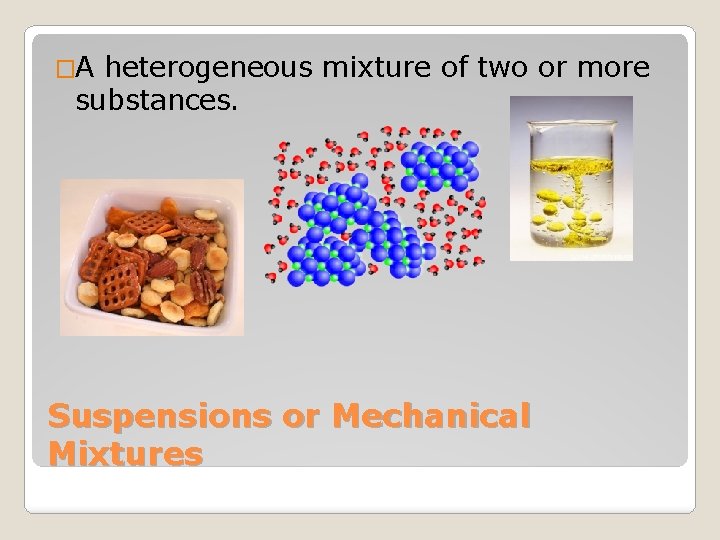
�A heterogeneous mixture of two or more substances. Suspensions or Mechanical Mixtures

�A homogenous mixture of two or more substances. Solutions

�A solution in which the maximum amount of solvent has been dissolved. Saturated Solution
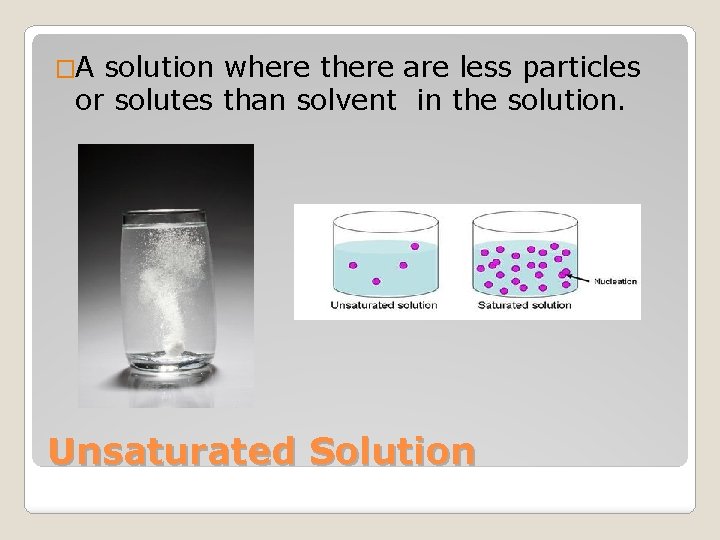
�A solution where there are less particles or solutes than solvent in the solution. Unsaturated Solution
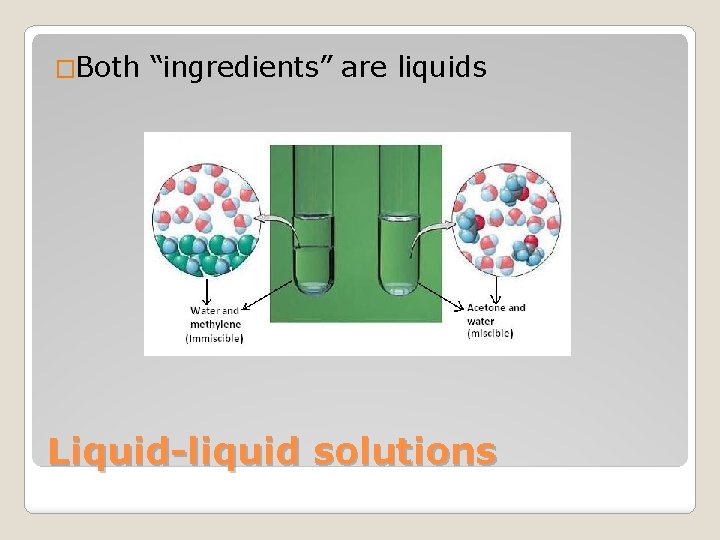
�Both “ingredients” are liquids Liquid-liquid solutions
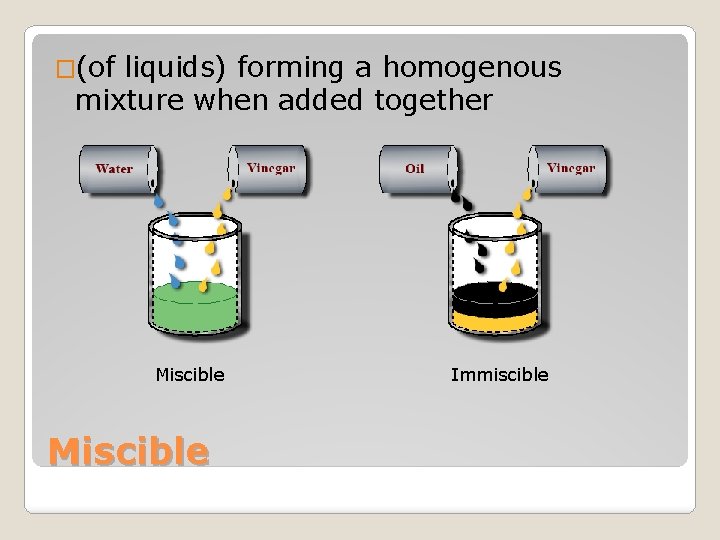
�(of liquids) forming a homogenous mixture when added together Miscible Immiscible

�“ingredients” are different. One being a solid and one being a liquid. Solid-liquid solutions

�“ingredients” are different. One being a gas and one being a liquid. Gas-liquid solutions

�Both “ingredients” are gasses. Gas-gas solutions

�When a gas occupies room between solid atoms. Palladium Gas-solid solutions Iron
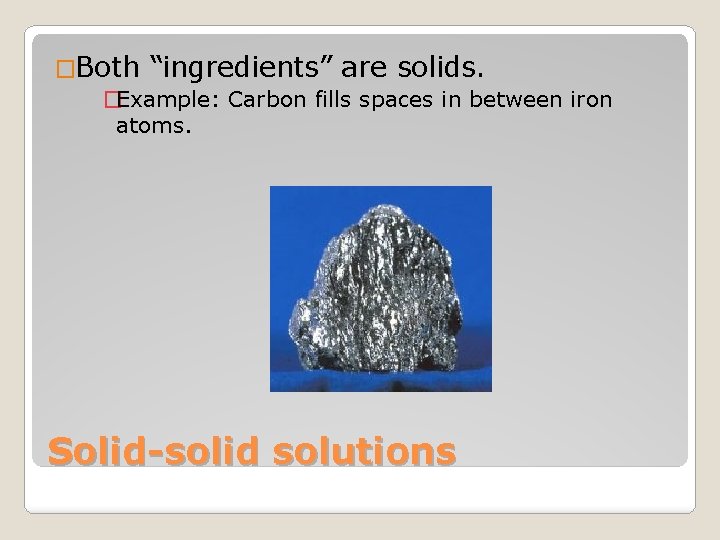
�Both “ingredients” are solids. �Example: Carbon fills spaces in between iron atoms. Solid-solid solutions
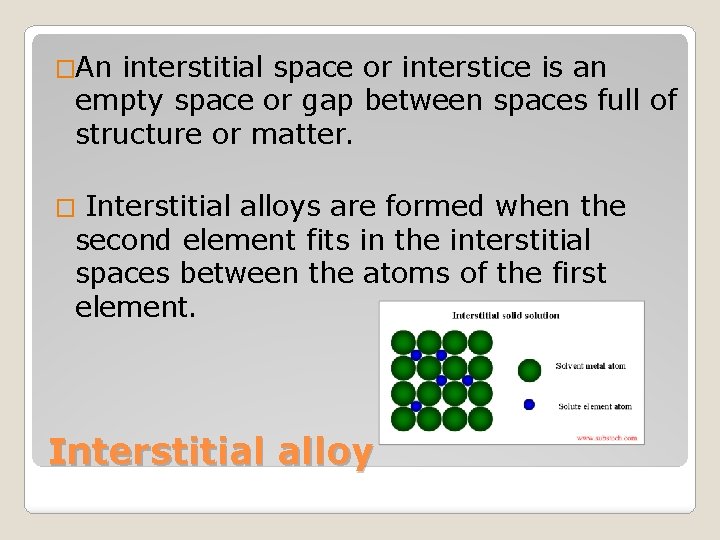
�An interstitial space or interstice is an empty space or gap between spaces full of structure or matter. Interstitial alloys are formed when the second element fits in the interstitial spaces between the atoms of the first element. � Interstitial alloy
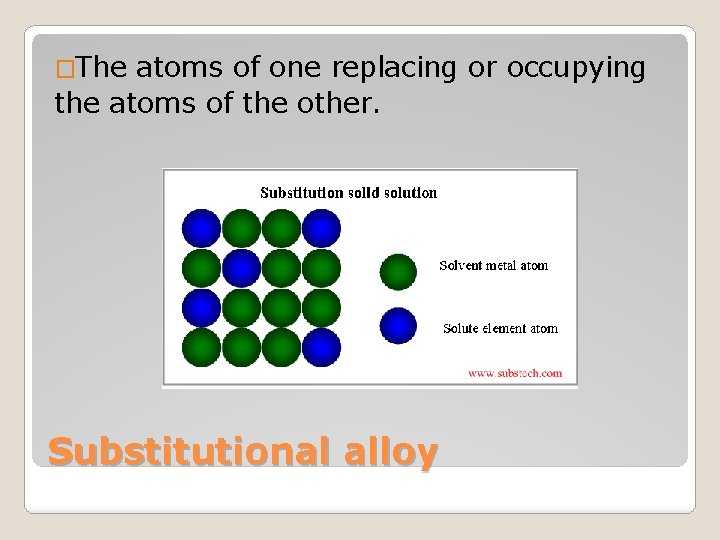
�The atoms of one replacing or occupying the atoms of the other. Substitutional alloy
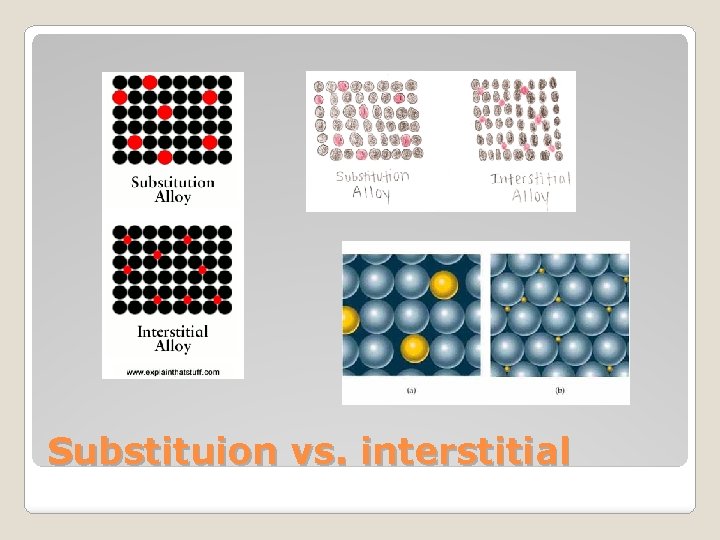
Substituion vs. interstitial
- Slides: 16