6 1 Types of Chemical Reactions Synthesis Combination

6. 1 – Types of Chemical Reactions
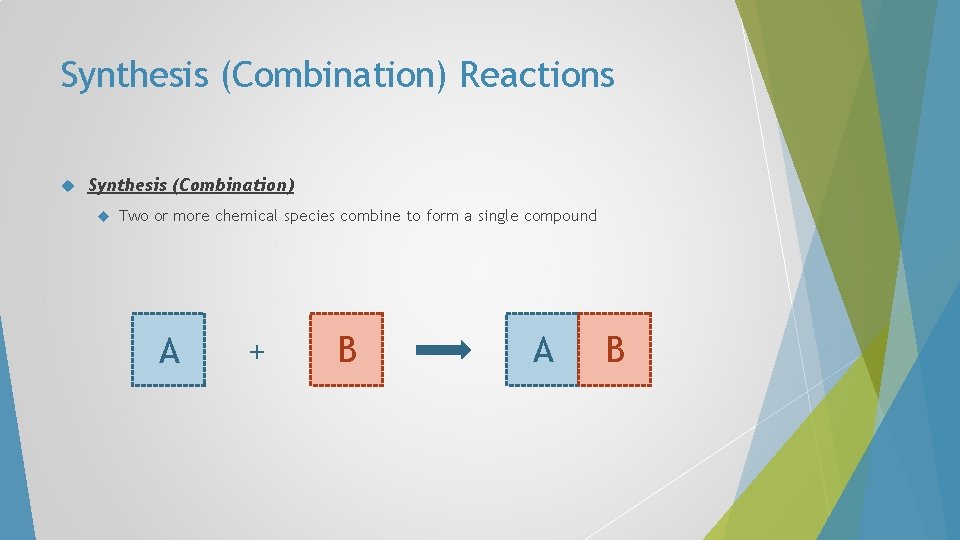
Synthesis (Combination) Reactions Synthesis (Combination) Two or more chemical species combine to form a single compound A + B A B
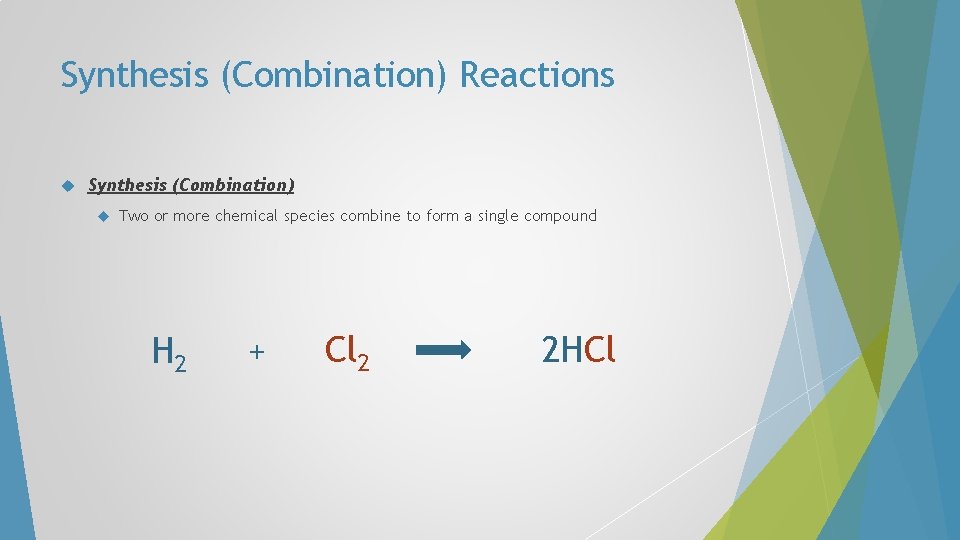
Synthesis (Combination) Reactions Synthesis (Combination) Two or more chemical species combine to form a single compound H 2 + Cl 2 2 HCl
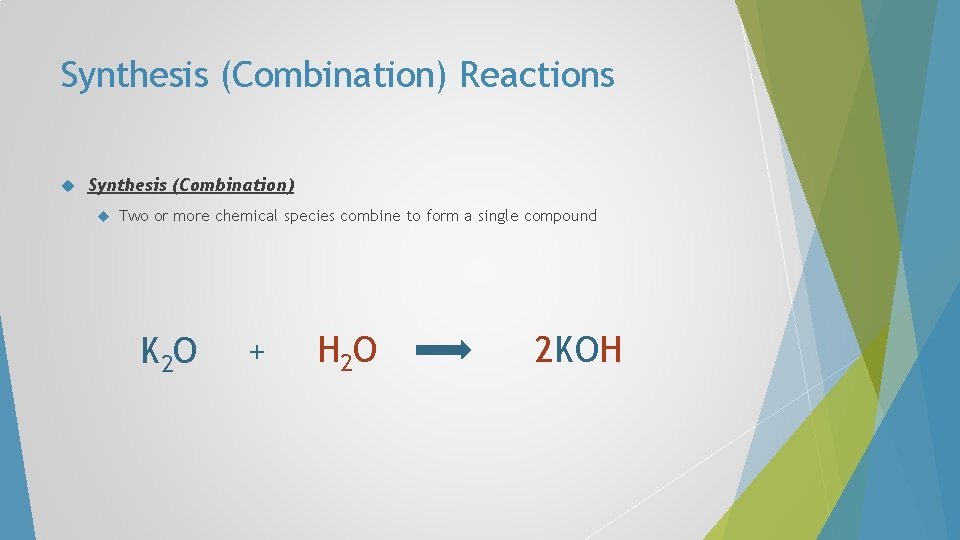
Synthesis (Combination) Reactions Synthesis (Combination) Two or more chemical species combine to form a single compound K 2 O + H 2 O 2 KOH
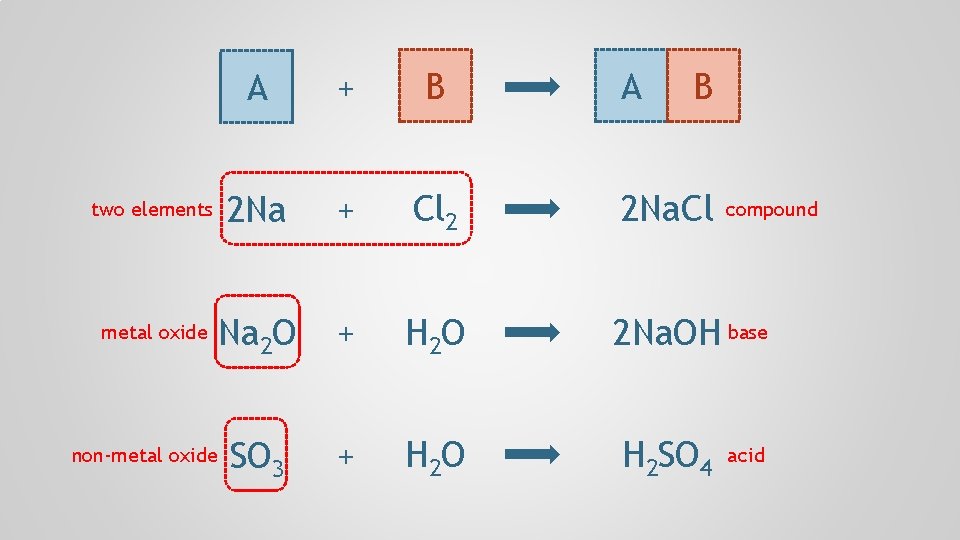
A + B two elements 2 Na + Cl 2 2 Na. Cl metal oxide Na 2 O + H 2 O 2 Na. OH base SO 3 + H 2 O H 2 SO 4 non-metal oxide A B compound acid
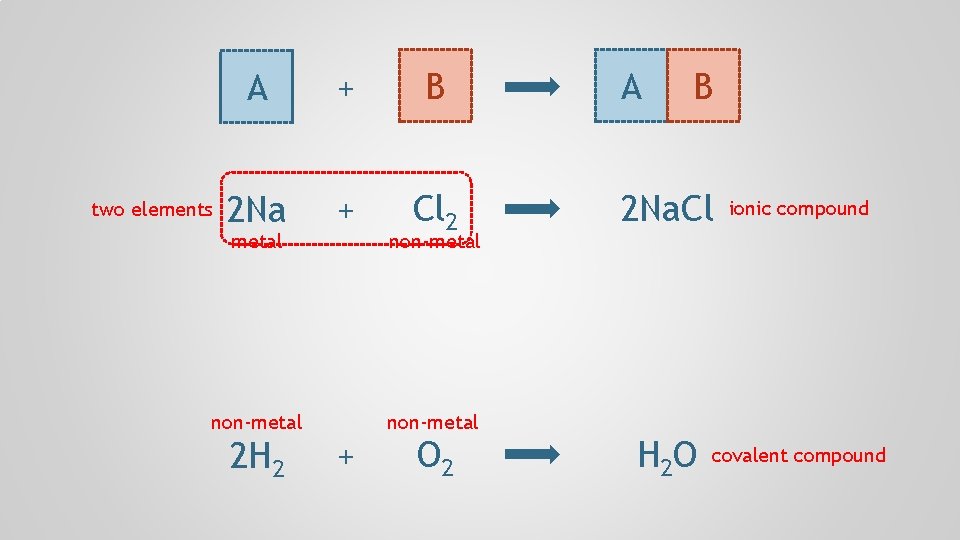
two elements A + B 2 Na + Cl 2 metal non-metal 2 H 2 non-metal + O 2 A B 2 Na. Cl H 2 O ionic compound covalent compound
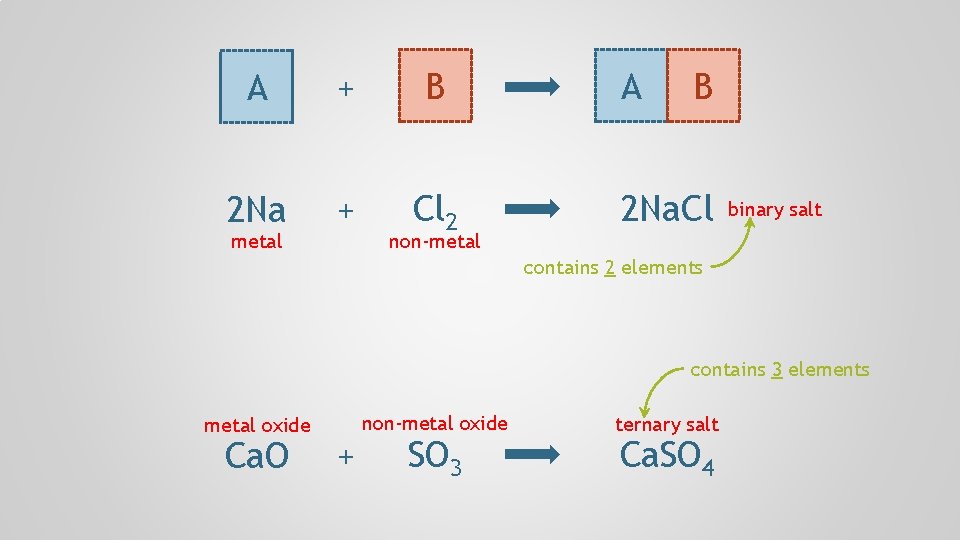
A + B 2 Na + Cl 2 metal non-metal A B 2 Na. Cl binary salt contains 2 elements contains 3 elements metal oxide Ca. O + non-metal oxide SO 3 ternary salt Ca. SO 4

Synthesis (Combination) Reactions Exothermic Describes a process that releases energy to the surroundings, most commonly in the form of heat and/or light
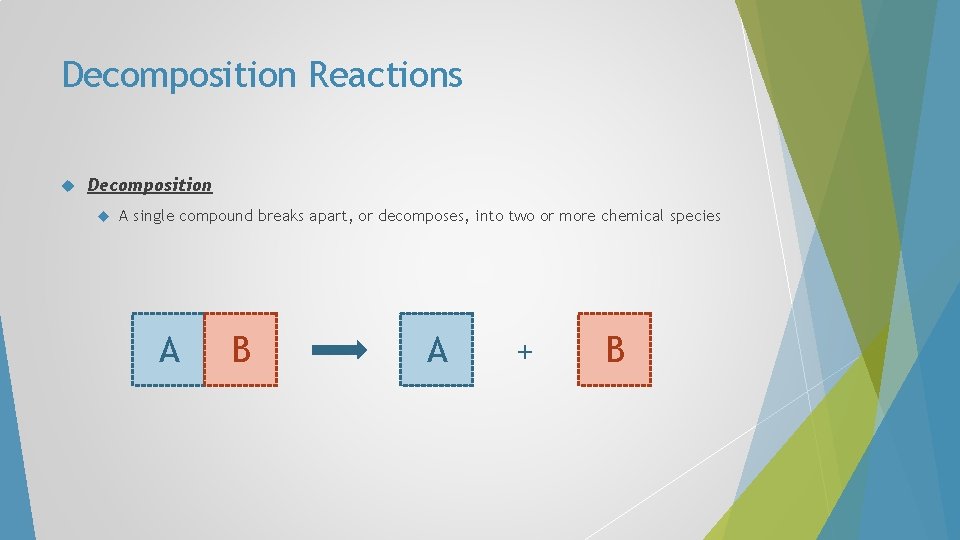
Decomposition Reactions Decomposition A single compound breaks apart, or decomposes, into two or more chemical species A B A + B

Decomposition Reactions Endothermic Describes a process that absorbs energy from the surroundings, most commonly in the form of heat and/or light
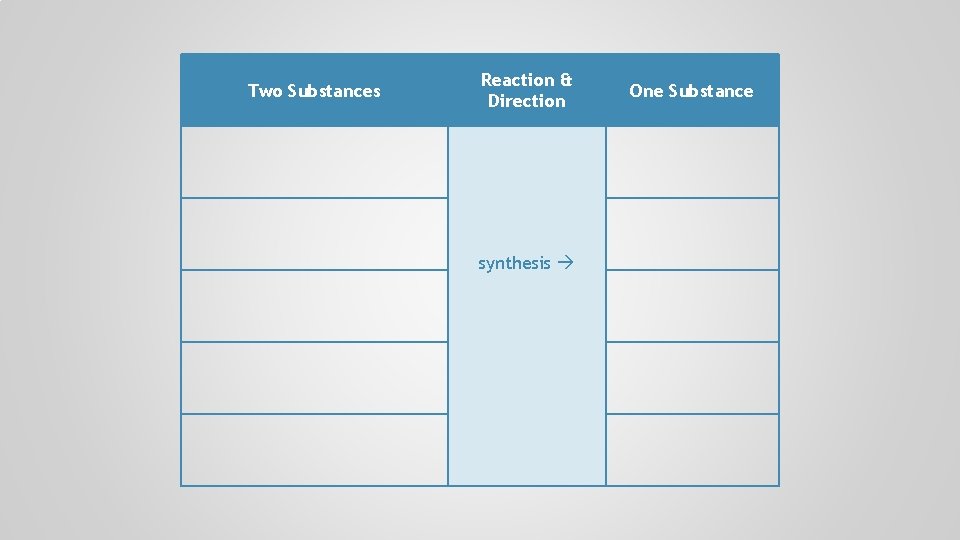
Two Substances Reaction & Direction One Substance
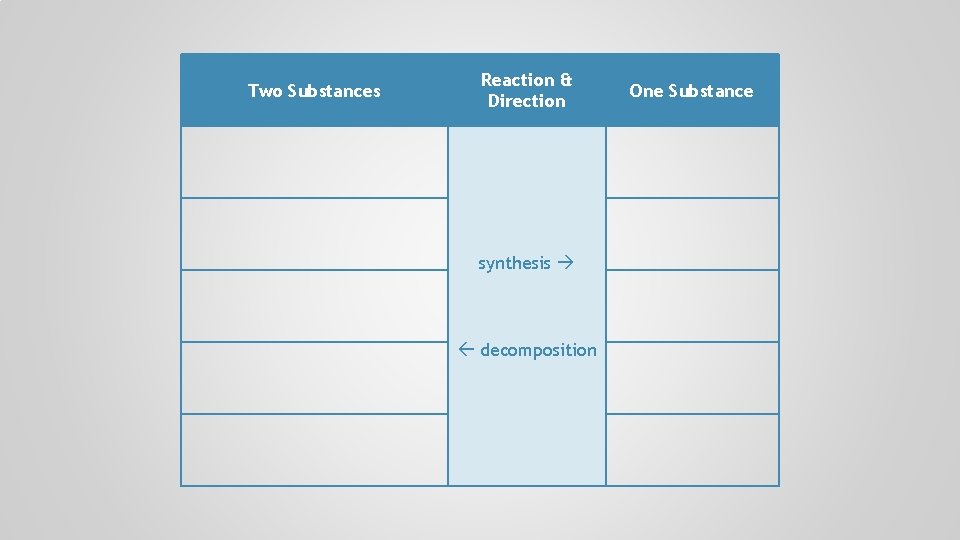
Two Substances Reaction & Direction synthesis decomposition One Substance
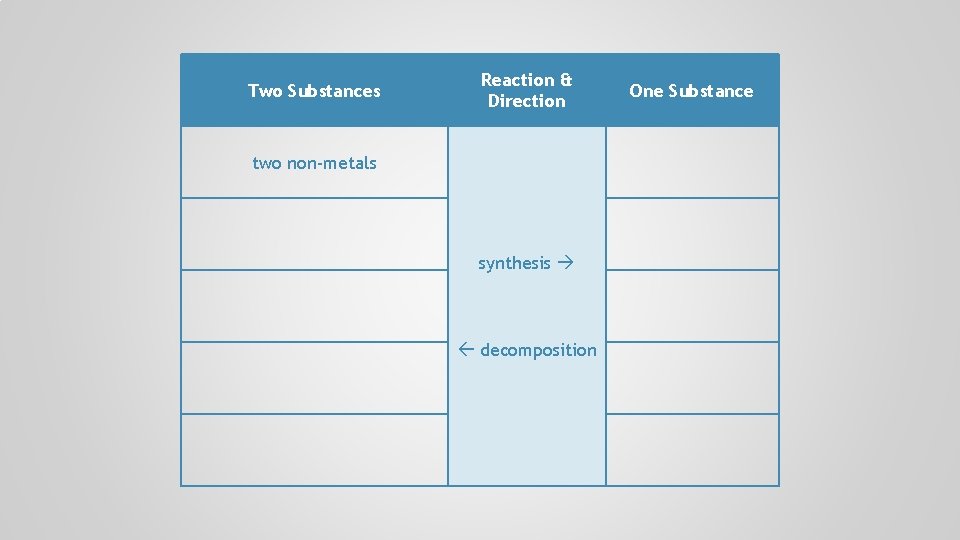
Two Substances Reaction & Direction synthesis decomposition One Substance
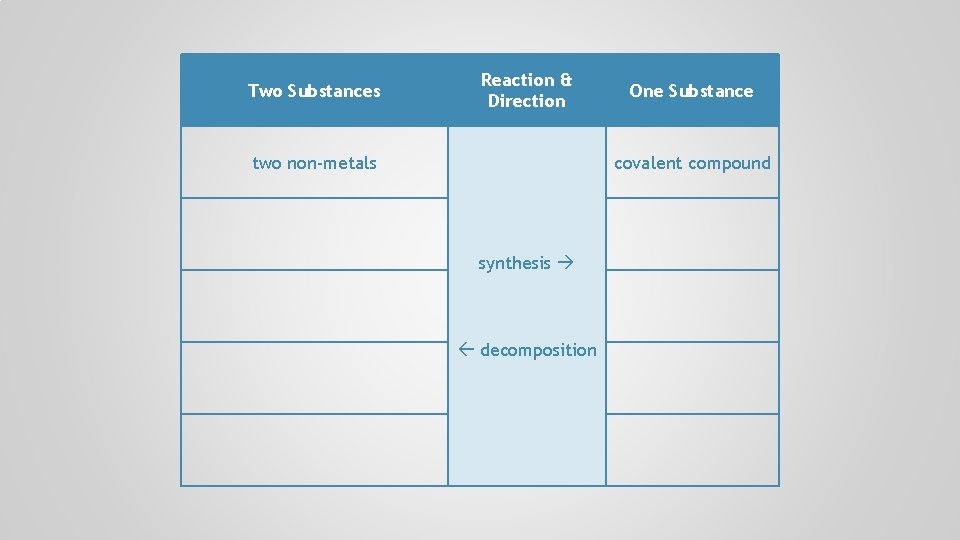
Two Substances Reaction & Direction two non-metals synthesis decomposition One Substance
Two Substances Reaction & Direction two non-metals One Substance covalent compound synthesis decomposition
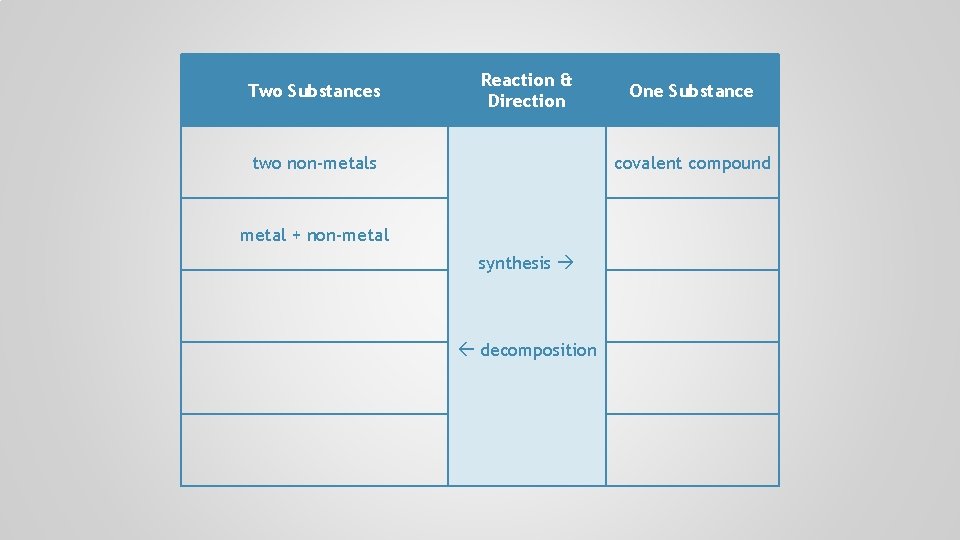
Two Substances Reaction & Direction two non-metals One Substance covalent compound metal + non-metal synthesis decomposition
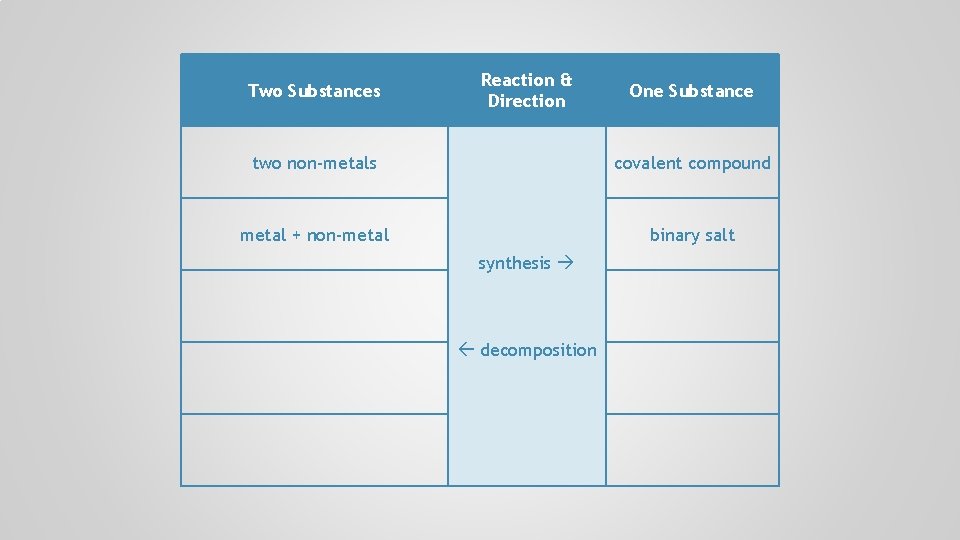
Two Substances Reaction & Direction One Substance two non-metals covalent compound metal + non-metal binary salt synthesis decomposition
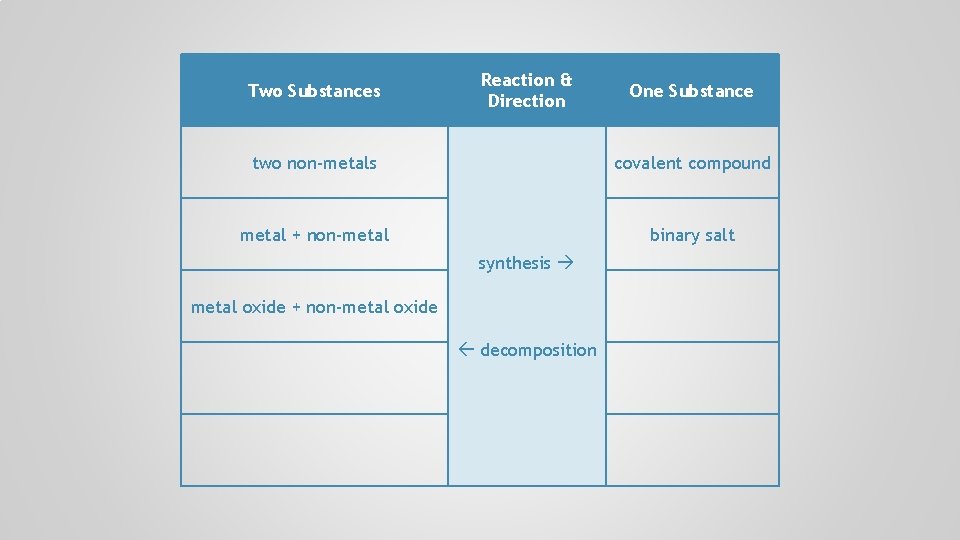
Two Substances Reaction & Direction One Substance two non-metals covalent compound metal + non-metal binary salt synthesis metal oxide + non-metal oxide decomposition
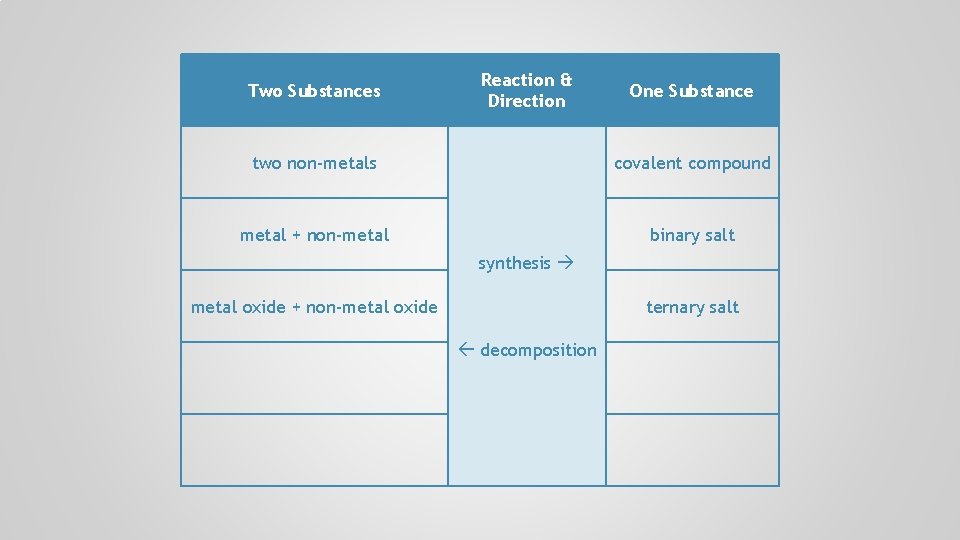
Two Substances Reaction & Direction One Substance two non-metals covalent compound metal + non-metal binary salt synthesis metal oxide + non-metal oxide ternary salt decomposition
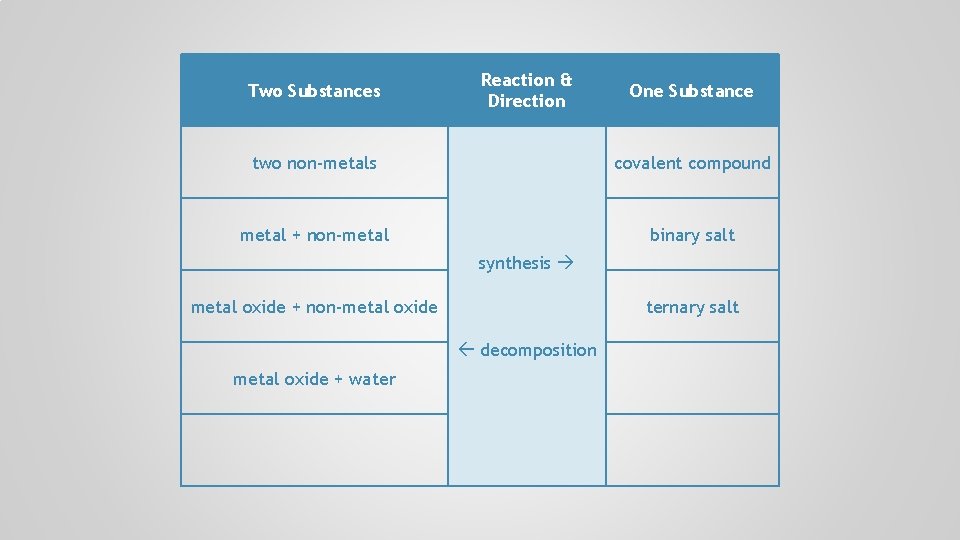
Two Substances Reaction & Direction One Substance two non-metals covalent compound metal + non-metal binary salt synthesis metal oxide + non-metal oxide ternary salt decomposition metal oxide + water
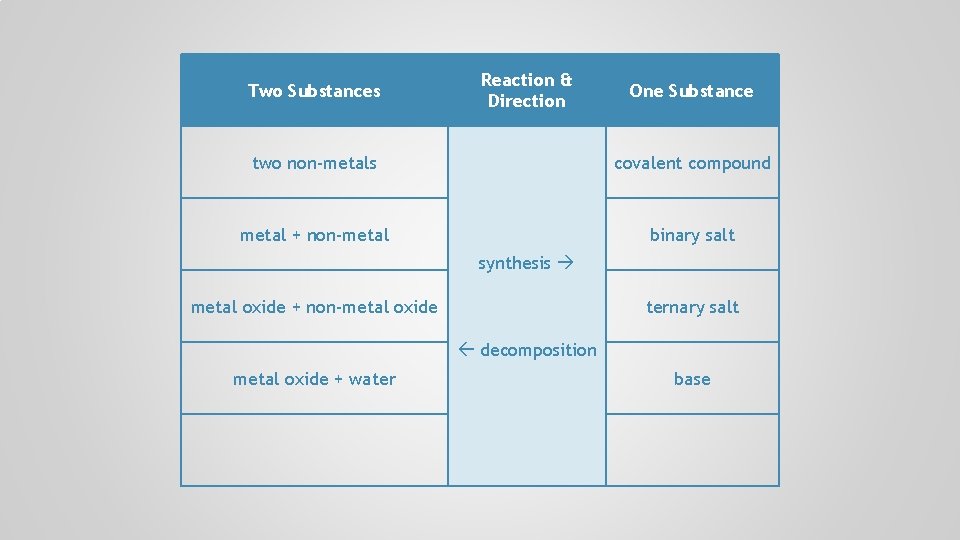
Two Substances Reaction & Direction One Substance two non-metals covalent compound metal + non-metal binary salt synthesis metal oxide + non-metal oxide ternary salt decomposition metal oxide + water base
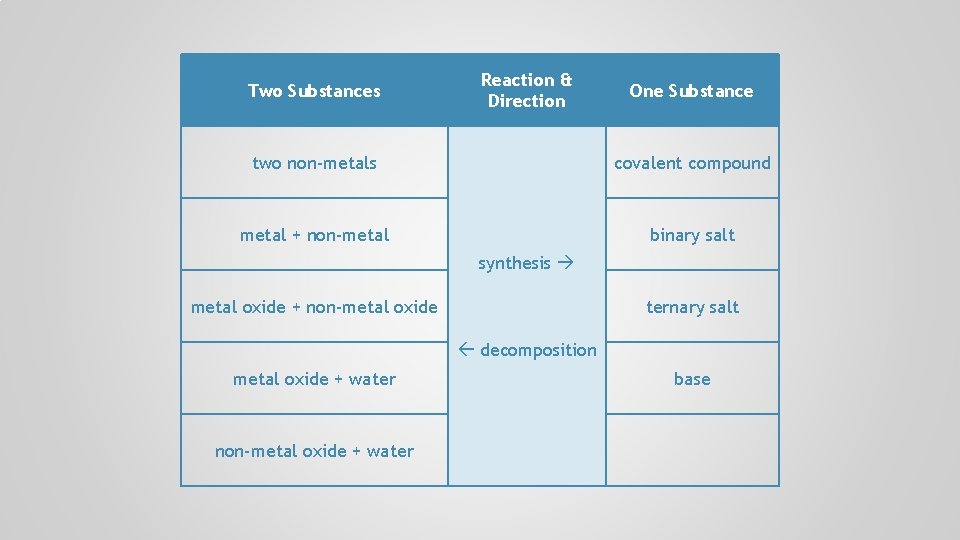
Two Substances Reaction & Direction One Substance two non-metals covalent compound metal + non-metal binary salt synthesis metal oxide + non-metal oxide ternary salt decomposition metal oxide + water non-metal oxide + water base
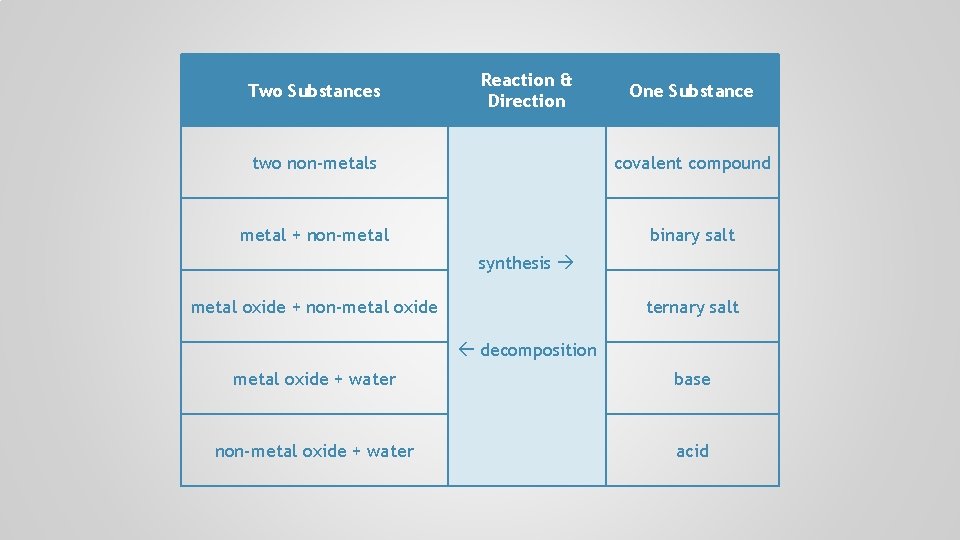
Two Substances Reaction & Direction One Substance two non-metals covalent compound metal + non-metal binary salt synthesis metal oxide + non-metal oxide ternary salt decomposition metal oxide + water base non-metal oxide + water acid
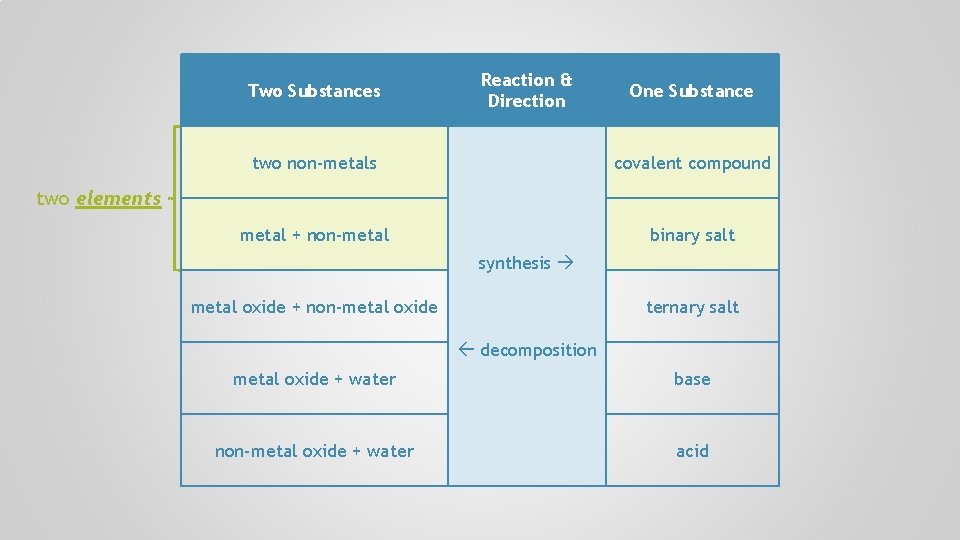
Two Substances Reaction & Direction One Substance two non-metals covalent compound metal + non-metal binary salt two elements synthesis metal oxide + non-metal oxide ternary salt decomposition metal oxide + water base non-metal oxide + water acid
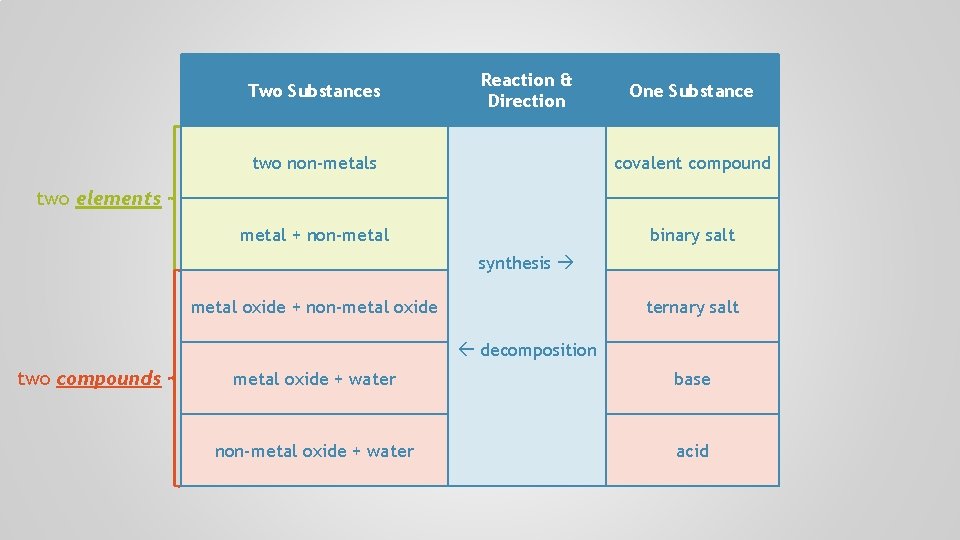
Two Substances Reaction & Direction One Substance two non-metals covalent compound metal + non-metal binary salt two elements synthesis metal oxide + non-metal oxide ternary salt decomposition two compounds metal oxide + water base non-metal oxide + water acid
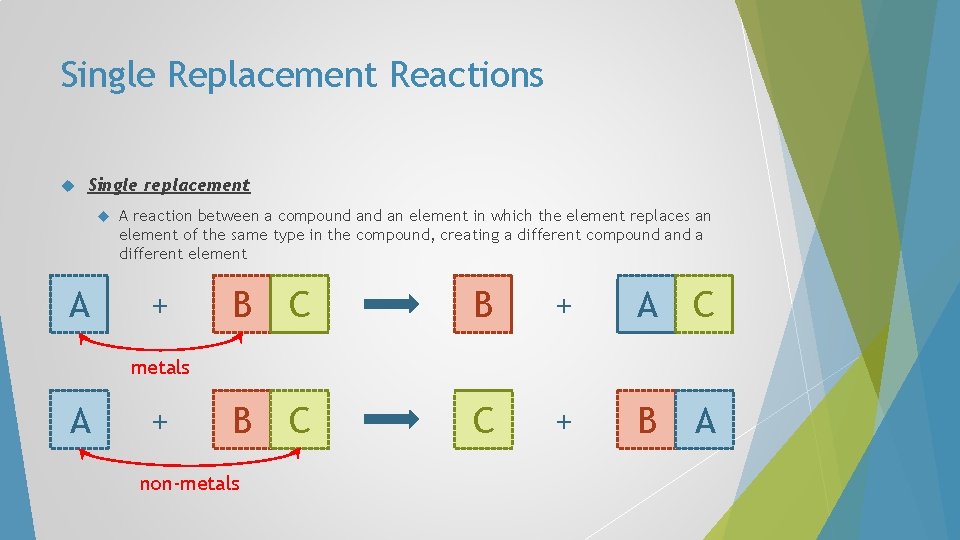
Single Replacement Reactions Single replacement A A reaction between a compound an element in which the element replaces an element of the same type in the compound, creating a different compound a different element + B C B + A C B C C + B A metals A + non-metals
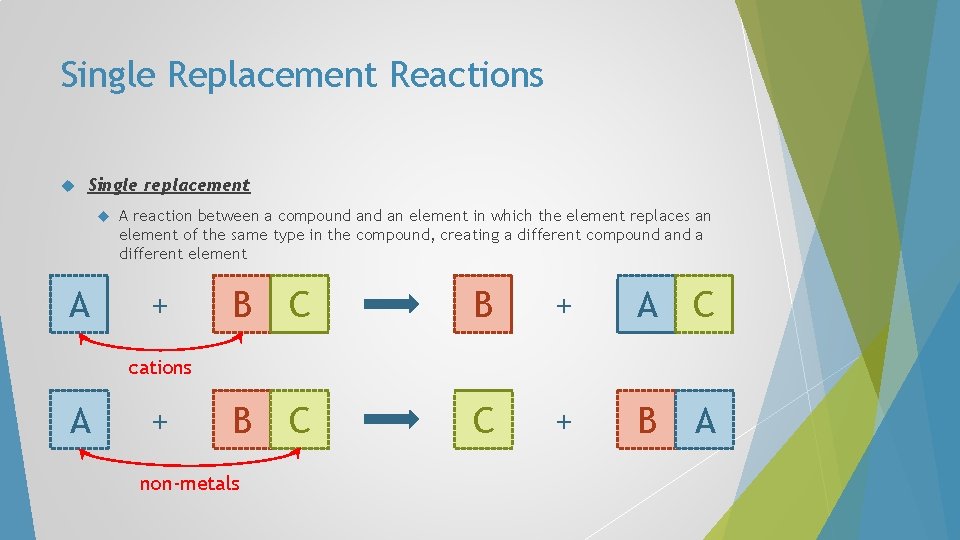
Single Replacement Reactions Single replacement A A reaction between a compound an element in which the element replaces an element of the same type in the compound, creating a different compound a different element + B C B + A C B C C + B A cations A + non-metals
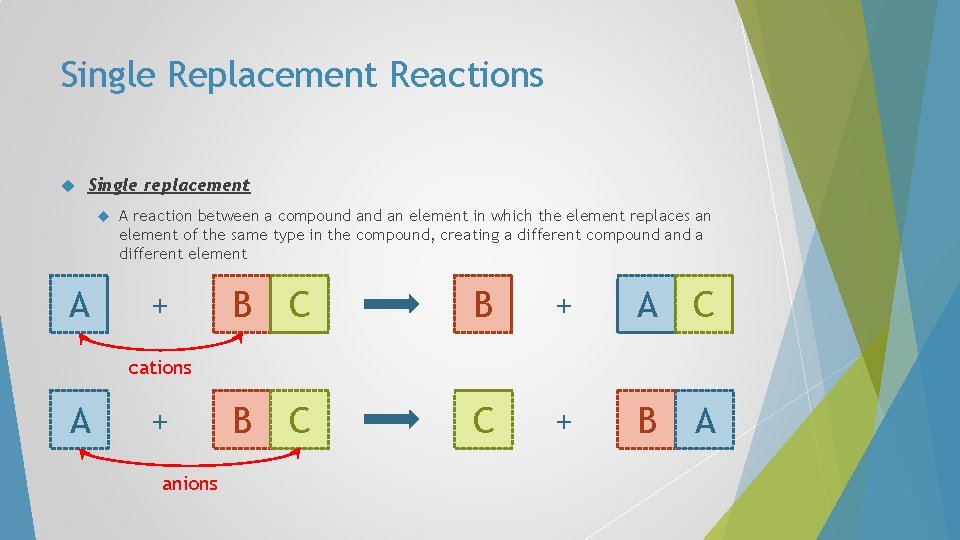
Single Replacement Reactions Single replacement A A reaction between a compound an element in which the element replaces an element of the same type in the compound, creating a different compound a different element + B C B + A C B C C + B A cations A + anions
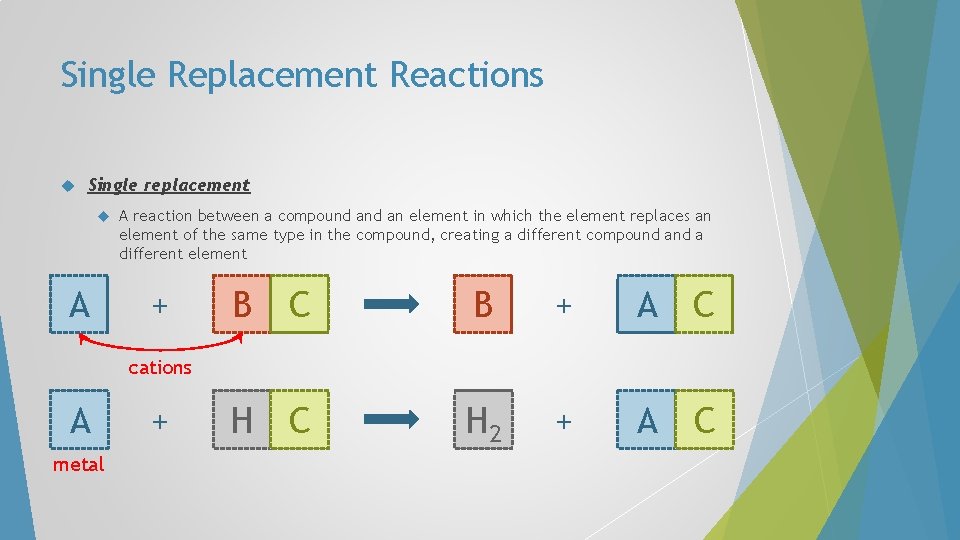
Single Replacement Reactions Single replacement A A reaction between a compound an element in which the element replaces an element of the same type in the compound, creating a different compound a different element + C B + A C H 2 + A C B cations A metal +
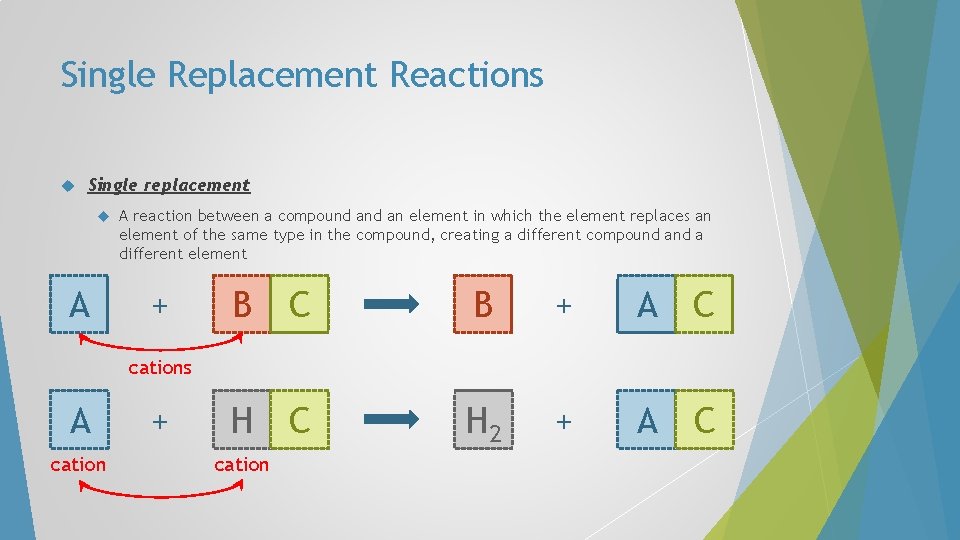
Single Replacement Reactions Single replacement A A reaction between a compound an element in which the element replaces an element of the same type in the compound, creating a different compound a different element + C B + A C H 2 + A C B cations A cation + cation

Single Replacement Reactions In single-replacement reactions, like replaces like – metals replace metals and hydrogen, non-metals replace nonmetals
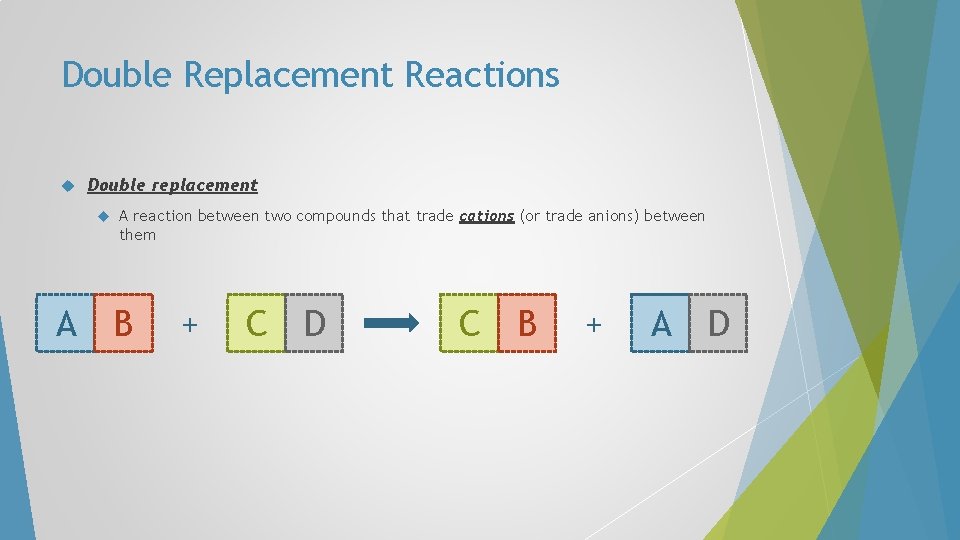
Double Replacement Reactions Double replacement A A reaction between two compounds that trade cations (or trade anions) between them B + C D C B + A D
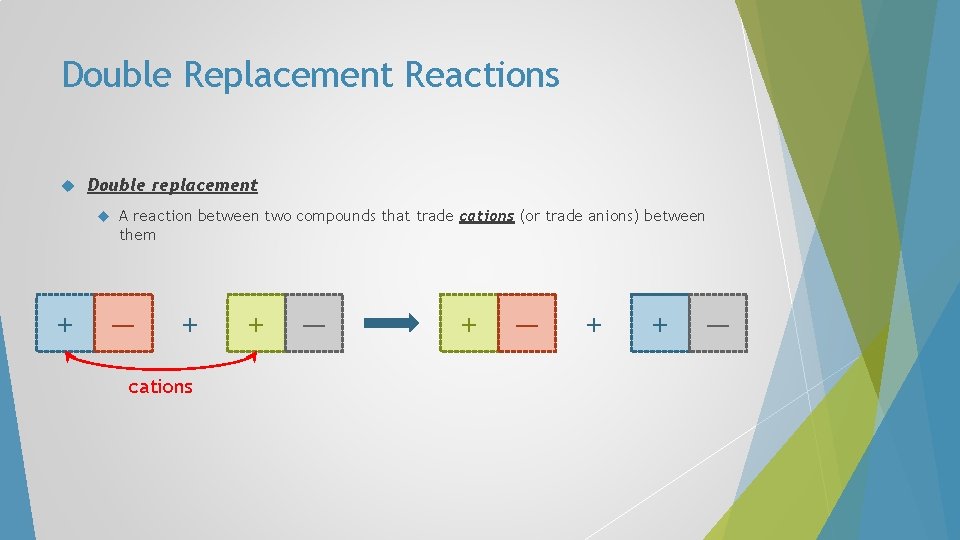
Double Replacement Reactions Double replacement A reaction between two compounds that trade cations (or trade anions) between them + — + cations + — + + —
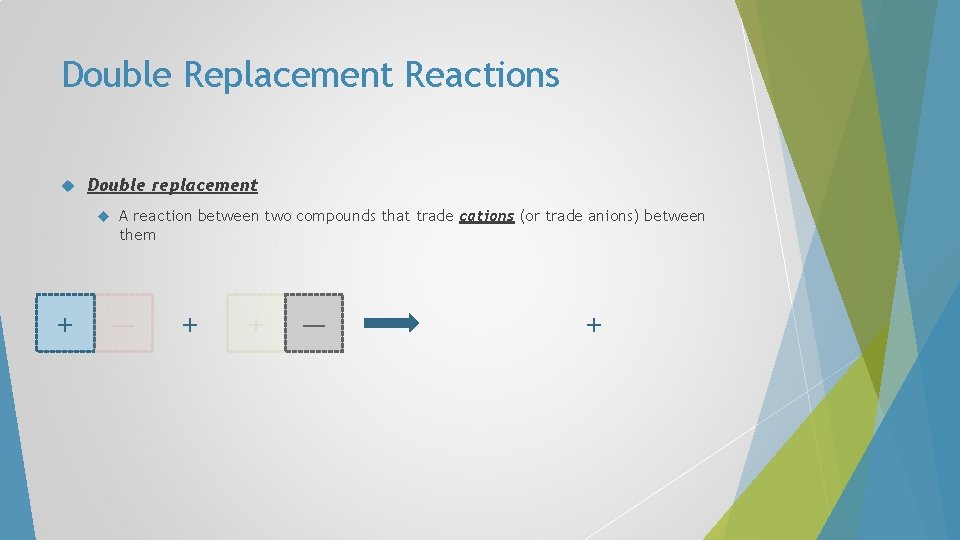
Double Replacement Reactions Double replacement A reaction between two compounds that trade cations (or trade anions) between them + — +
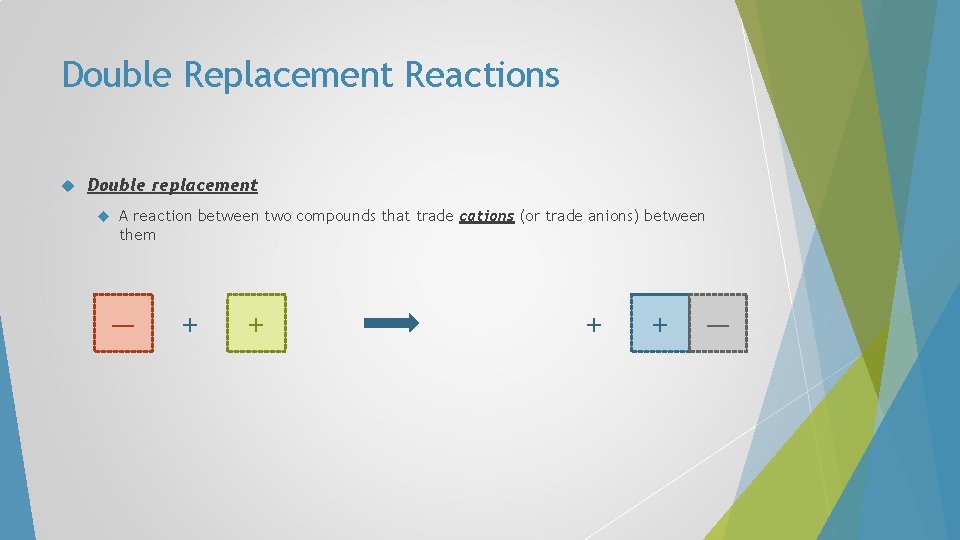
Double Replacement Reactions Double replacement A reaction between two compounds that trade cations (or trade anions) between them — + + —
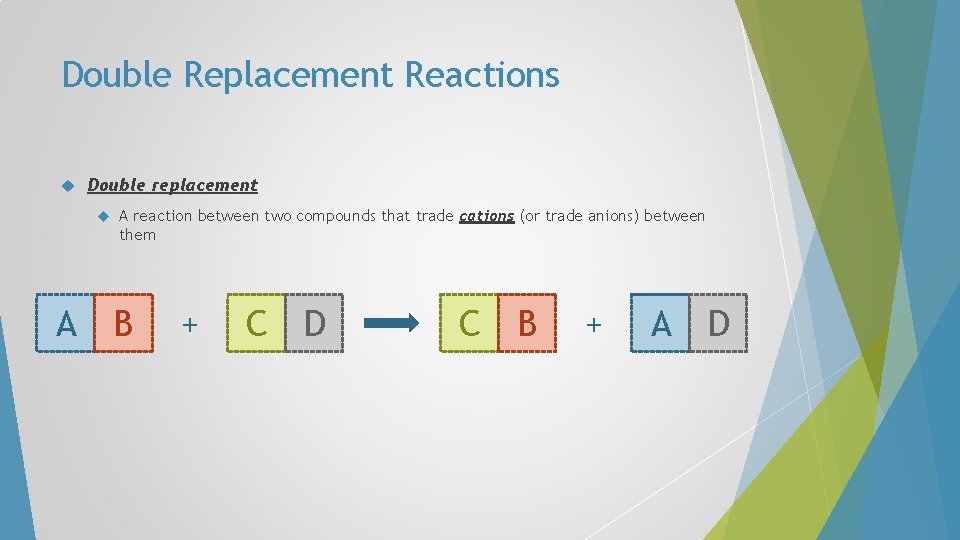
Double Replacement Reactions Double replacement A A reaction between two compounds that trade cations (or trade anions) between them B + C D C B + A D
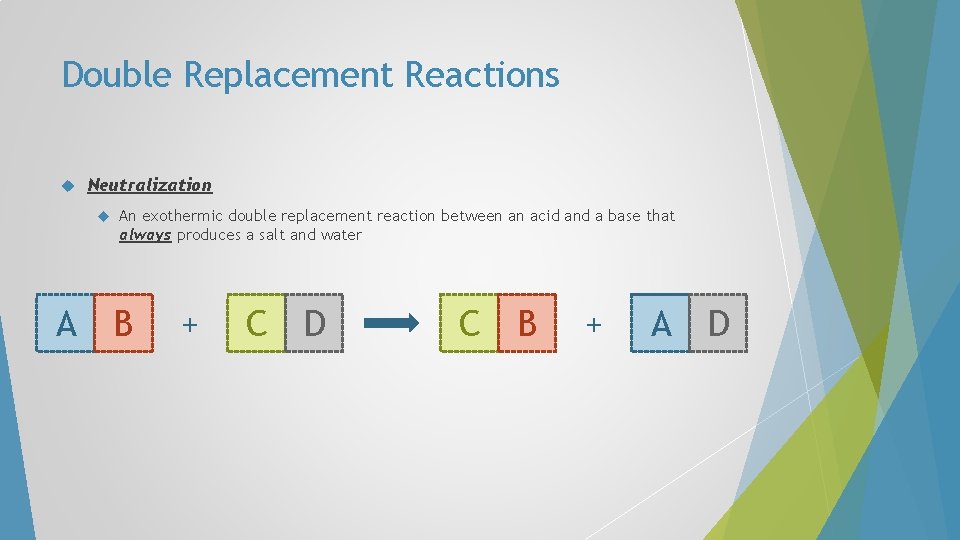
Double Replacement Reactions Neutralization A An exothermic double replacement reaction between an acid and a base that always produces a salt and water B + C D C B + A D
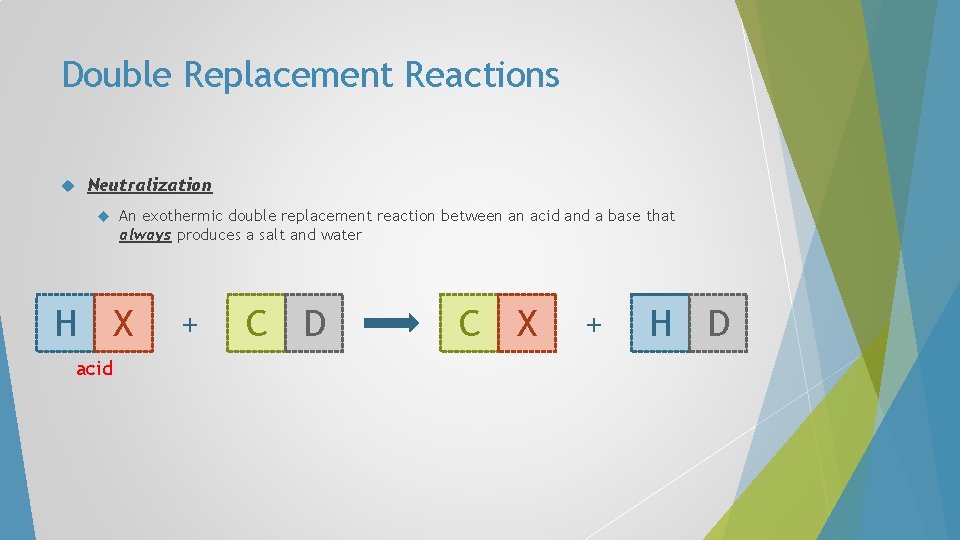
Double Replacement Reactions Neutralization An exothermic double replacement reaction between an acid and a base that always produces a salt and water H X acid + C D C X + H D
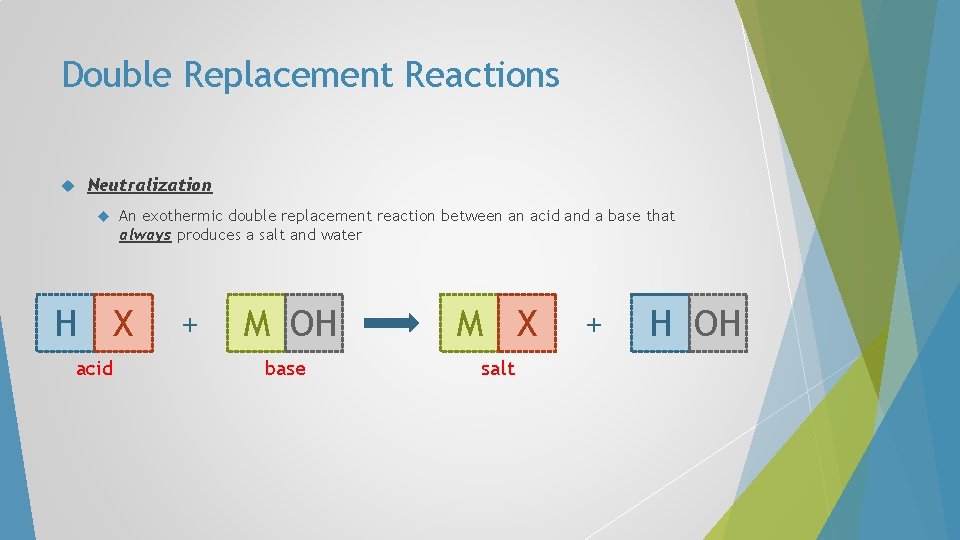
Double Replacement Reactions Neutralization An exothermic double replacement reaction between an acid and a base that always produces a salt and water H X acid + M OH M X base salt + H OH
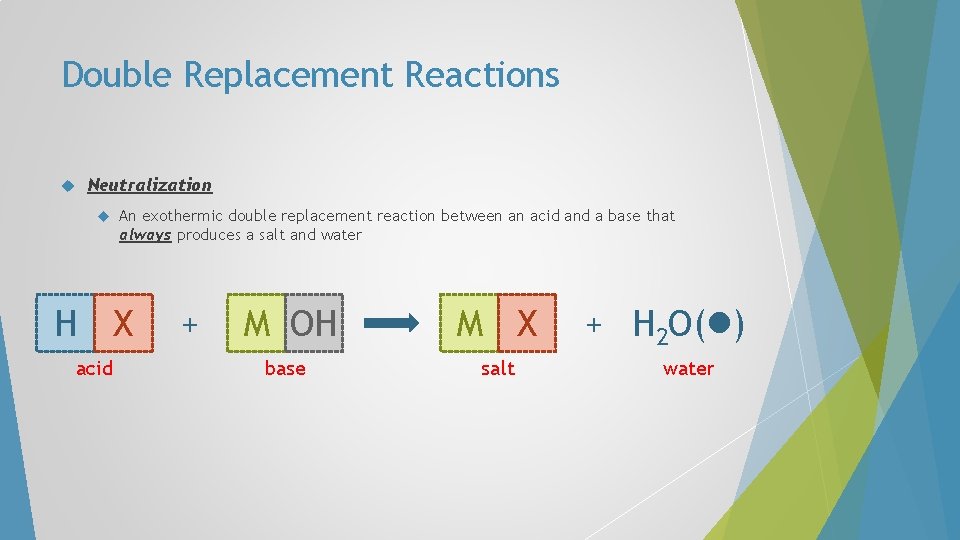
Double Replacement Reactions Neutralization An exothermic double replacement reaction between an acid and a base that always produces a salt and water H X acid + M OH M X base salt + H 2 O( ) water

Double Replacement Reactions Neutralization An exothermic double replacement reaction between an acid and a base that always produces a salt and water H X acid + M OH base MX(aq) + H 2 O( ) salt water
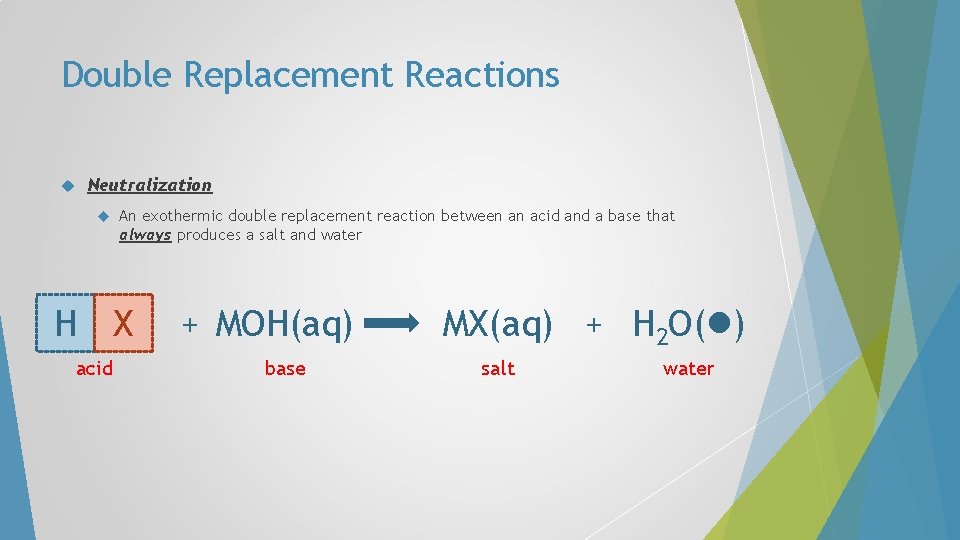
Double Replacement Reactions Neutralization An exothermic double replacement reaction between an acid and a base that always produces a salt and water H X acid + MOH(aq) base MX(aq) + H 2 O( ) salt water
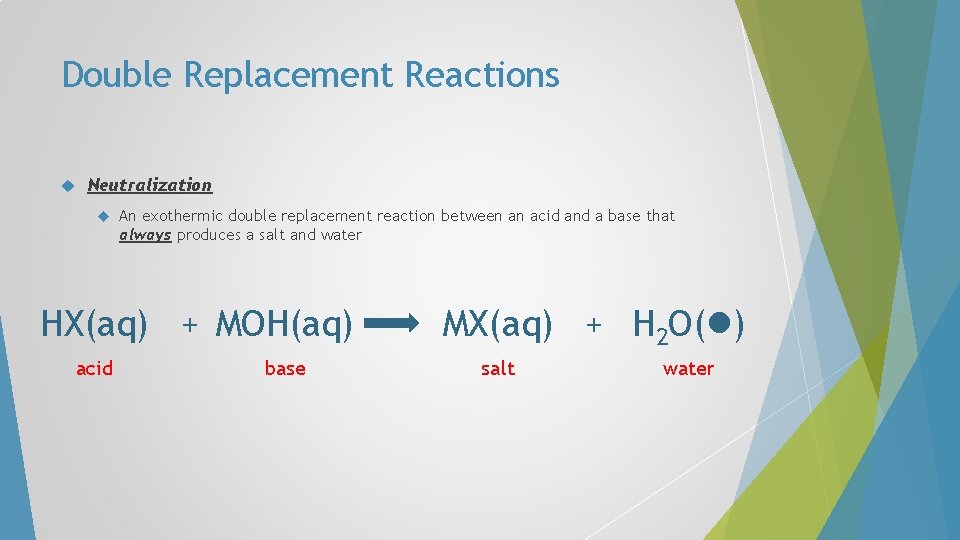
Double Replacement Reactions Neutralization An exothermic double replacement reaction between an acid and a base that always produces a salt and water HX(aq) + MOH(aq) acid base MX(aq) + H 2 O( ) salt water

Combustion Reactions Combustion A rapid and exothermic reaction between oxygen and a fuel, producing carbon dioxide and water, along with other oxides, depending on the composition of the fuel Fuel is a hydrocarbon CXHY + O 2 CO 2 + H 2 O Fuel is a carbohydrate CXHYOZ + O 2 CO 2 + H 2 O
- Slides: 44