530 352 Materials Selection Lecture 26 High Temperature










- Slides: 19

530. 352 Materials Selection Lecture #26 High Temperature Creep - II Tuesday November 15 th, 2005

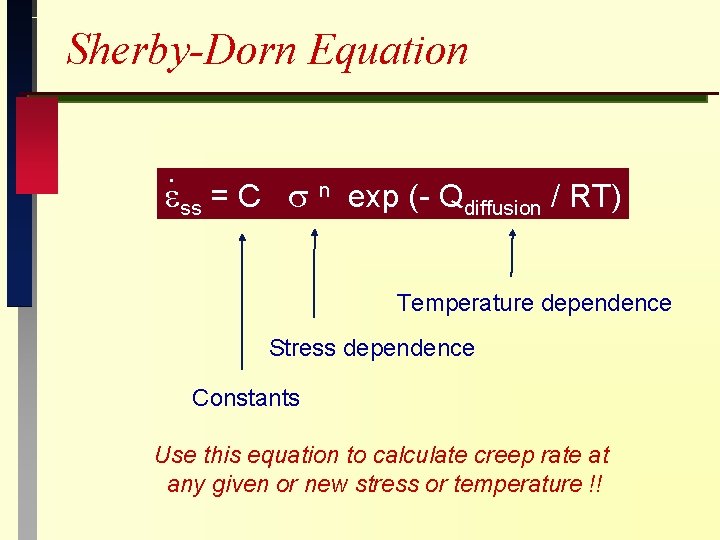
Sherby-Dorn Equation. ss = C n exp (- Qdiffusion / RT) Temperature dependence Stress dependence Constants Use this equation to calculate creep rate at any given or new stress or temperature !!

Example of creep based design: Ni-base superalloys that are used for jet turbine applications exhibit Qcreep = 320 k. J/mol and n=5. • What is the creep rate at 925 o. C and 350 MPa if C=1. 7 x 10 -7 and R=8. 314 J/mol-o. C ? • What would the creep rate be if the stress were increased by 25 MPa ? • What would the creep rate be if the temperature were increased by 25 o. C ? • If your boss wanted to increase the operating temperature by 50 o. C, how much would you have to decrease the stress to maintain the same creep rate ?

Creep rate; T=925 o. C and s=350 MPa. ss = C n exp (- Q / RT) . ss = 1. 7 x 10 -7 3505 exp( -320, 000/8. 314 x 1198 K ) = 1. 7 x 10 -7 x 5. 25 x 1012 = 10 -8 sec-1 x 11. 1 x 10 -15

Is 10 -8 sec-1 fast ? Is short for a service life but long for a graduate student -- must extrapolate from short tests to long times !!

Increasing by 25 MPa : . ss-1 = C n exp (- Q / RT 1) . ss-2 = C 2 n exp (- Q / RT 2) . . 1 / 2 = ( / 2 )n = (350/375)5 = 0. 708 . 2 = 1. 4 x 10 -8 sec

Increasing by 25 o. C: . ss-1 = C n exp (- Q / RT 1) . ss-2 = C 2 n exp (- Q / RT 2) 2 = 1. 92 x 10 -8 sec

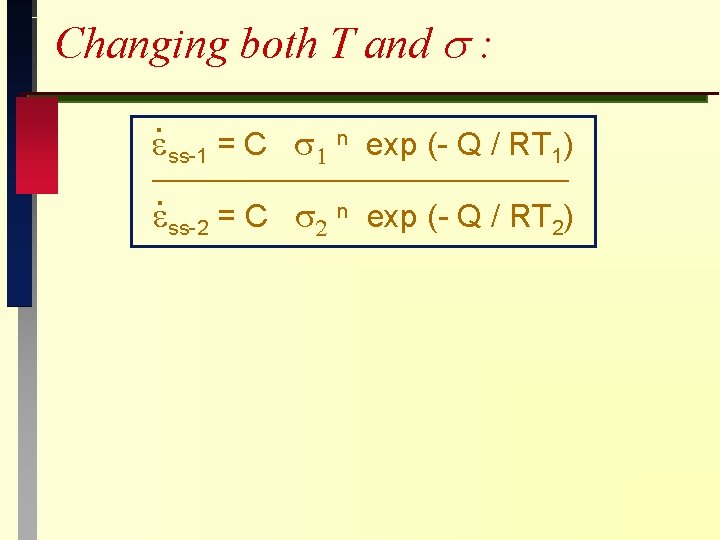
Changing both T and s : . ss-1 = C n exp (- Q / RT 1) . ss-2 = C 2 n exp (- Q / RT 2)

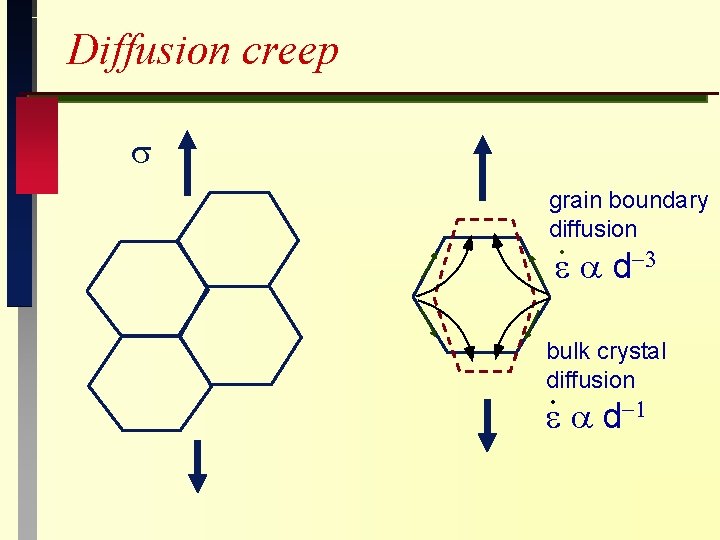
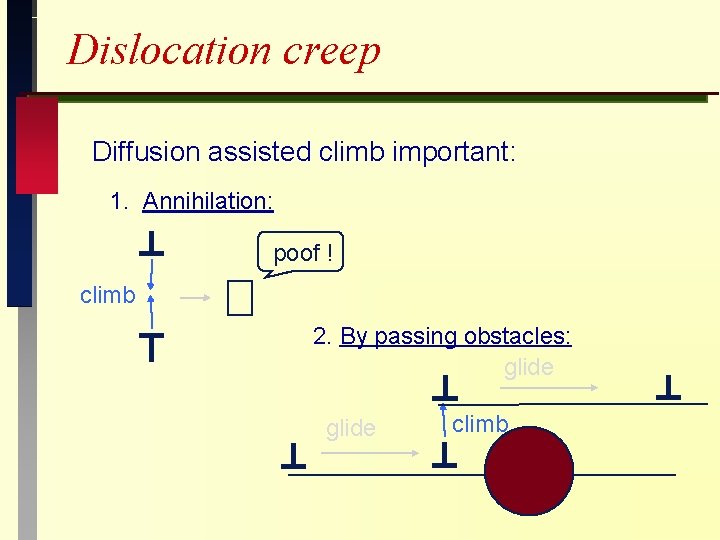
Creep Mechanisms (metals and ceramics) Diffusion creep Dislocation creep (power-law creep) Stress Relaxation Creep Fracture

Diffusion creep grain boundary diffusion d bulk crystal diffusion d

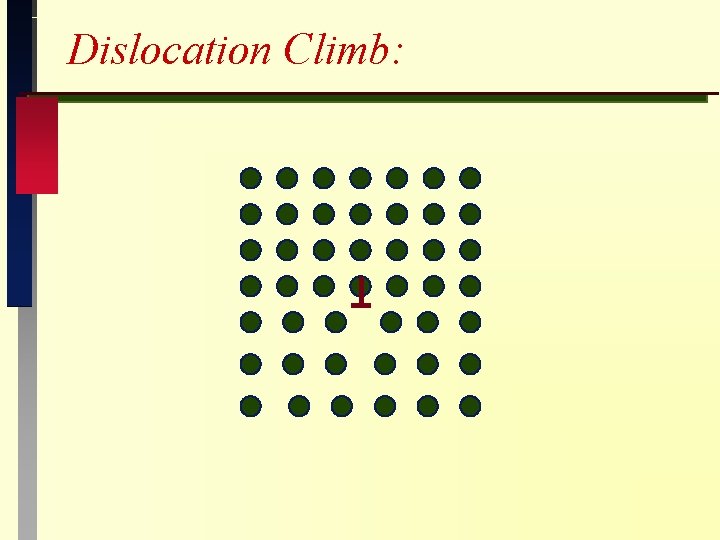
Dislocation creep Diffusion assisted climb important: 1. Annihilation: poof ! climb 2. By passing obstacles: glide climb

Dislocation Climb:

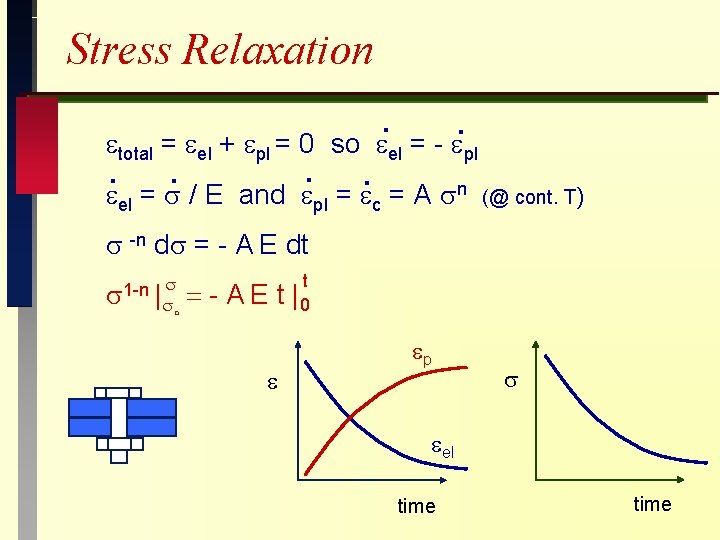
Stress Relaxation. total = el + pl = 0 so el . . . el = / E and pl . =- pl n = A c (@ cont. T) -n d = - A E dt 1 -n | = o AE t t |0 p el time

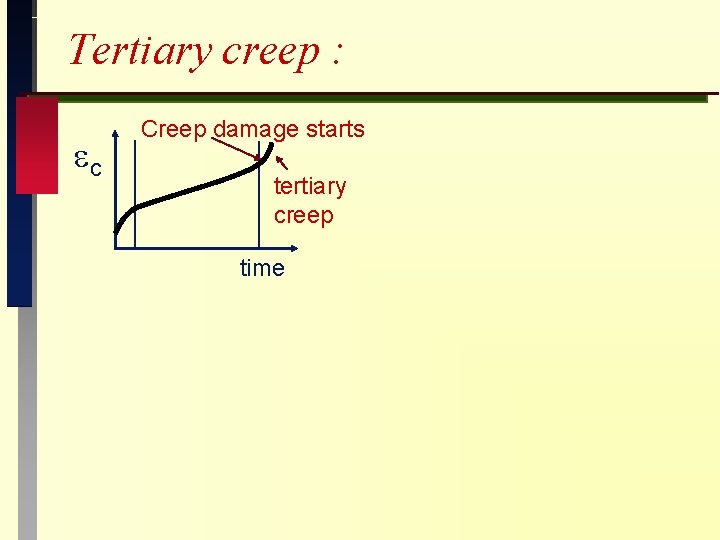
Tertiary creep : c Creep damage starts tertiary creep time

Design against creep (metals) Minimize T / Tmelting to slow diffusion, climb, and creep. Arrange for large grain sizes to slow diffusion. Use precipitates (oxide particles) and solid solutions to slow dislocations.

Creep in ceramics : Very little dislocation motion - mostly diffusion creep or something else. Glassy phases (oxides) that form at grain boundaries soften and high T and lead to grain boundary sliding.

Design against creep (ceramics) Similar to metals, reduce diffusion and dislocation motion, but must also. . . Reduce/control grain boundary phases.

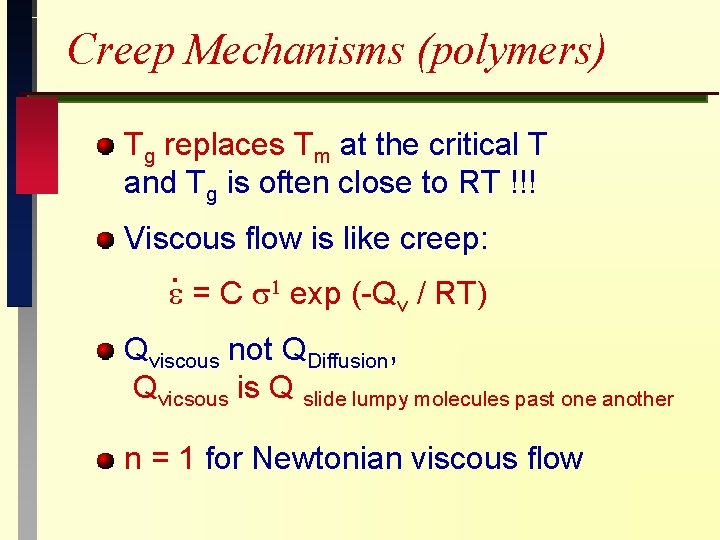
Creep Mechanisms (polymers) Tg replaces Tm at the critical T and Tg is often close to RT !!! Viscous flow is like creep: . = C exp (-Qv / RT) Qviscous not QDiffusion, Qvicsous is Q slide lumpy molecules past one another n = 1 for Newtonian viscous flow

Design Against Creep (polymers) Increased degree of cross-linking -> increased Tg and less creep. High molecular weight -> high viscosity -> low creep rate. Crystalline polymers better than glassy. Add fibers or particles to make composites !!