5 Quantum Mechanics G 482 Electricity Waves Photons





- Slides: 35

5 Quantum Mechanics G 482 Electricity, Waves & Photons 2. 5. 1 Energy of A Photon 2. 5. 2 The Photoelectric Effect 2. 5. 3 Wave. Particle Duality 2. 5. 4 Energy Levels in Atoms H 158/H 558 Mr Powell 2012 Index

Introduction. . The aim of this module is to introduce the concept of quantum behaviour. How do we know that light is a wave? The evidence for this comes from diffraction of light. However, this wave-like behaviour cannot explain how light interacts with electrons in a metal. A revolutionary model of light (photon model), developed by Max Planck and Albert Einstein, is needed to describe the interaction of light with matter. Physicists expect symmetry in nature. If light can have a dual nature, then surely particles like the electron must also have a dual nature. We study the ideas developed by de Broglie. The final section looks briefly at the idea that electrons in atoms have discrete bond energies and they move between energy levels by either absorbing or by emitting photons. There are many opportunities to discuss how theories and models develop with the history of wave-particle duality. Mr Powell 2012 Index

Practical Skills are assessed using OCR set tasks. The practical work suggested below may be carried out as part of skill development. Centres are not required to carry out all of these experiments. This module does not lend itself to many experiments carried by the students. However, it does contain many revolutionary ideas and engaging students in discussions is vital when demonstrating some of the experiments. Use a GM tube to ‘count’ gamma ray photons. Determine the wavelength of light from different LEDs Demonstrate the photoelectric effect using a photocell or a negatively charged zinc plate connected to an electroscope. Observe ‘diffraction rings’ for light passing through a tiny hole. Demonstrate the diffraction of electrons by graphite. Observe emission line spectra from different discharge tubes. (A hand-held optical spectrometer can be used to observe Fraunhofer lines in daylight. Caution: Do not look directly at the Sun. ) Mr Powell 2012 Index

SOW Activities. . . Resources. . Points to Note… 1. 1. 2. 3. 4. Class experiment: Observe line spectra from gas discharge tubes with diffraction gratings, handheld optical spectrometers or spectrometer. Discuss implications of fixed specific colours for each element – relate colour to energy of photons and then consider how they could be produced Relate energy levels to specific energies and jumps between levels to photon energy, hence hf = E 1 – E 2 Discuss emission spectra and absorption spectra. Illustrate with stellar spectra and test identification with spectra from different stars 2. 3. 4. Gas discharge tubes e. g. hydrogen, sodium, mercury. Spectrometer or several hand held spectrometers Students to take role of electrons, standing at different positions in lab with distance in one direction to represent energy. They have to decide how they can behave to give line spectra This can be extended to providing energy in one direction and producing absorption spectra End of topic test 2. 3. 4. 5. Ideally hand held spectrometers for immediacy (caution: do not look directly at sun) Absorb Physics for A level Quantum Physics – Emission spectra There is much scope for chemistry students to relate this topic to their course with electron orbitals, the periodic table and flame test identification of elements The story of Helium being discovered in the Sun before on Earth is relevant and an interesting part of HSW Mr Powell 2012 Index

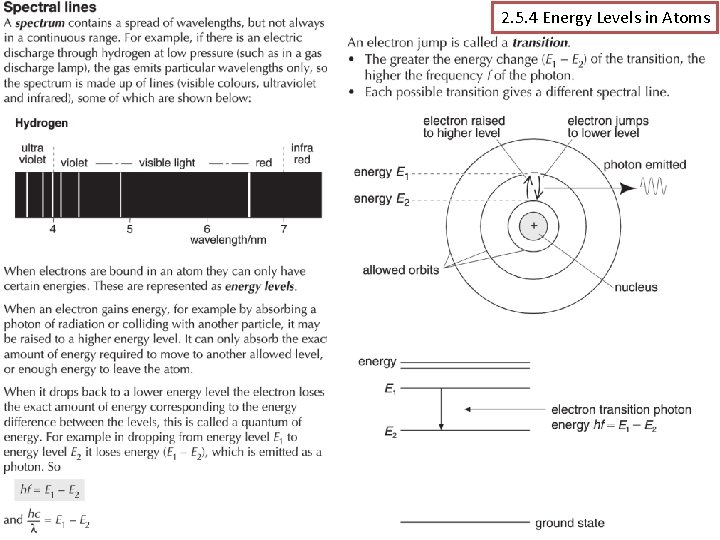
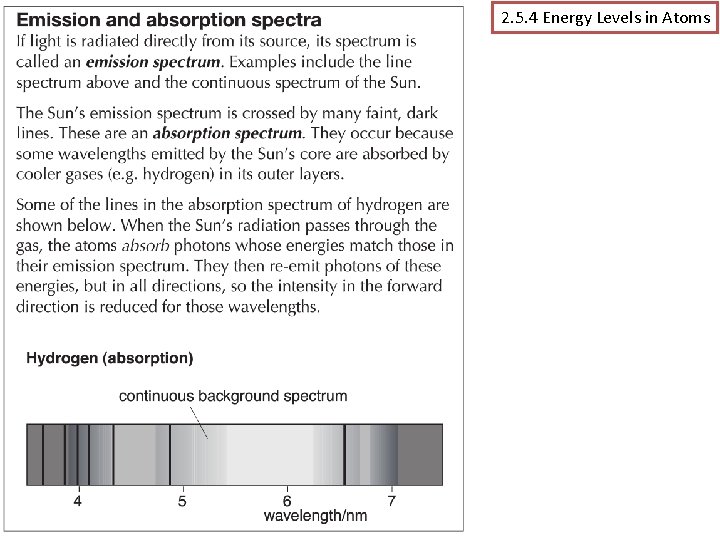
2. 5. 4 Energy Levels in Atoms (p 180 -185) Assessable learning outcomes. . (a) explain how spectral lines are evidence for the existence of discrete energy levels in isolated atoms, ie in a gas discharge lamp; (b) describe the origin of emission and absorption line spectra; (c) use the relationships hf = E 1 – E 2 and hc/ = E 1 – E 2 Mr Powell 2012 Index

2. 5. 4 Energy Levels in Atoms

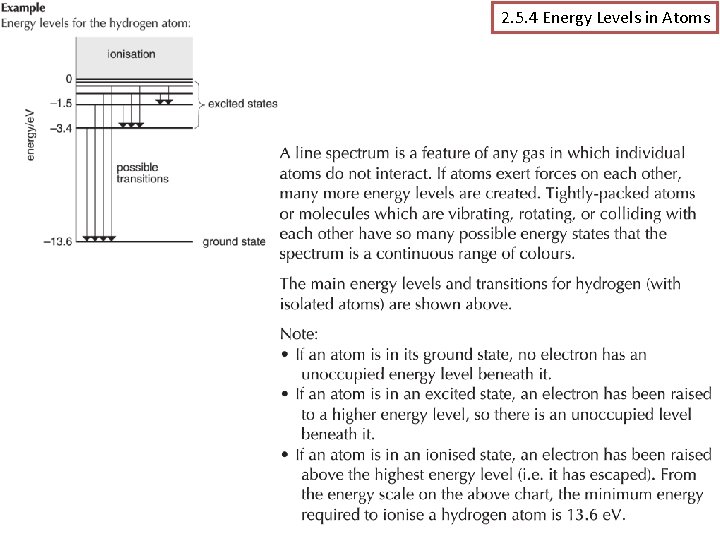
2. 5. 4 Energy Levels in Atoms

2. 5. 4 Energy Levels in Atoms

• How do we know which elements stars are made up from? • How do we know the age of stars? Mr Powell 2012 Index

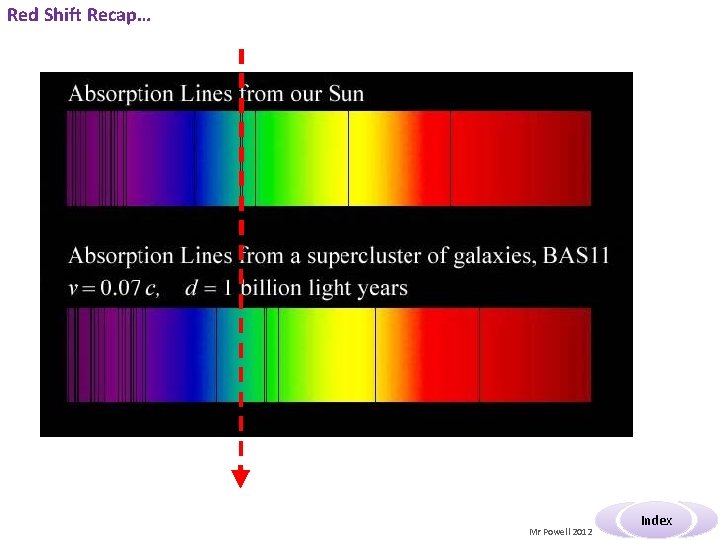
Red Shift Recap… Mr Powell 2012 Index

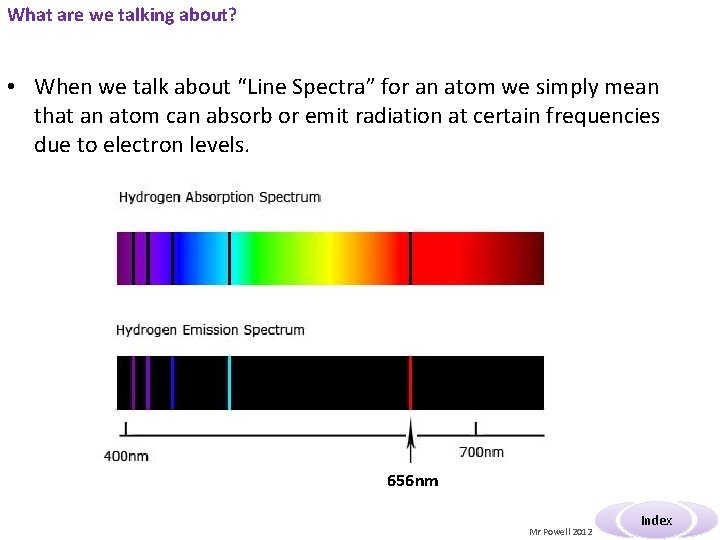
What are we talking about? • When we talk about “Line Spectra” for an atom we simply mean that an atom can absorb or emit radiation at certain frequencies due to electron levels. 656 nm Mr Powell 2012 Index

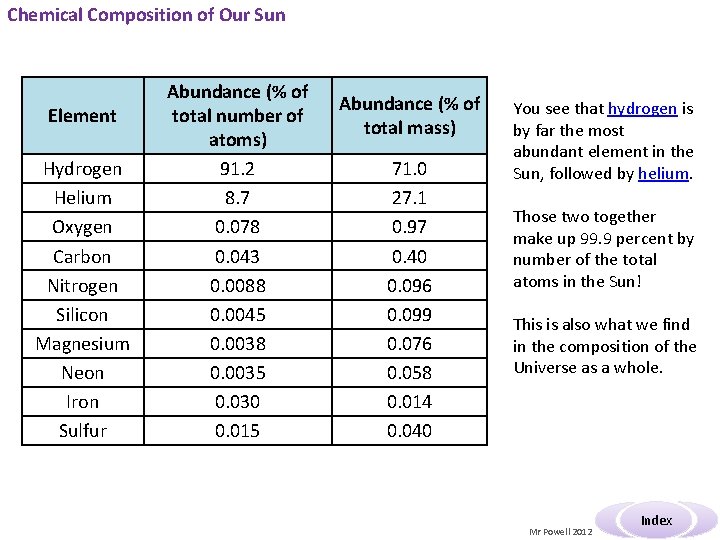
Chemical Composition of Our Sun Element Hydrogen Helium Oxygen Carbon Nitrogen Silicon Magnesium Neon Iron Sulfur Abundance (% of total number of atoms) 91. 2 8. 7 0. 078 0. 043 0. 0088 0. 0045 0. 0038 0. 0035 0. 030 0. 015 Abundance (% of total mass) 71. 0 27. 1 0. 97 0. 40 0. 096 0. 099 0. 076 0. 058 0. 014 0. 040 You see that hydrogen is by far the most abundant element in the Sun, followed by helium. Those two together make up 99. 9 percent by number of the total atoms in the Sun! This is also what we find in the composition of the Universe as a whole. Mr Powell 2012 Index

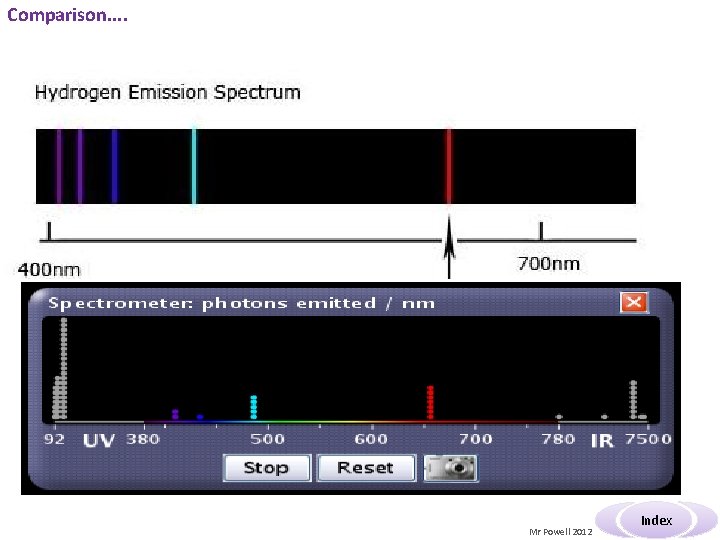
Comparison. . Mr Powell 2012 Index

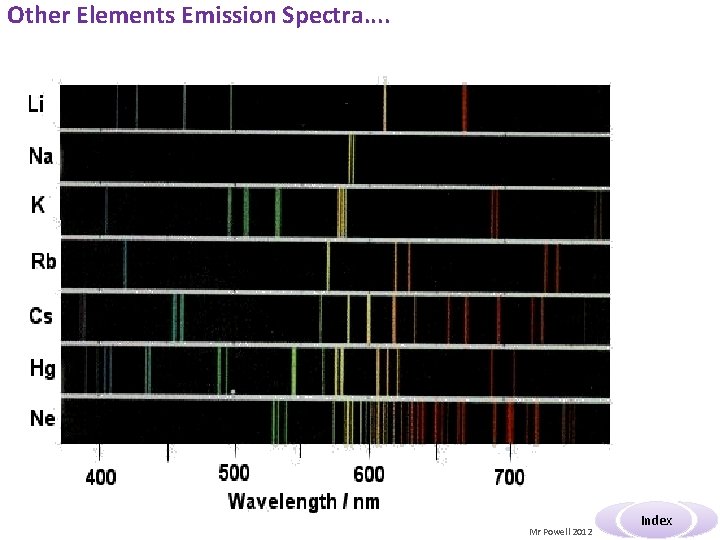
Other Elements Emission Spectra. . Mr Powell 2012 Index

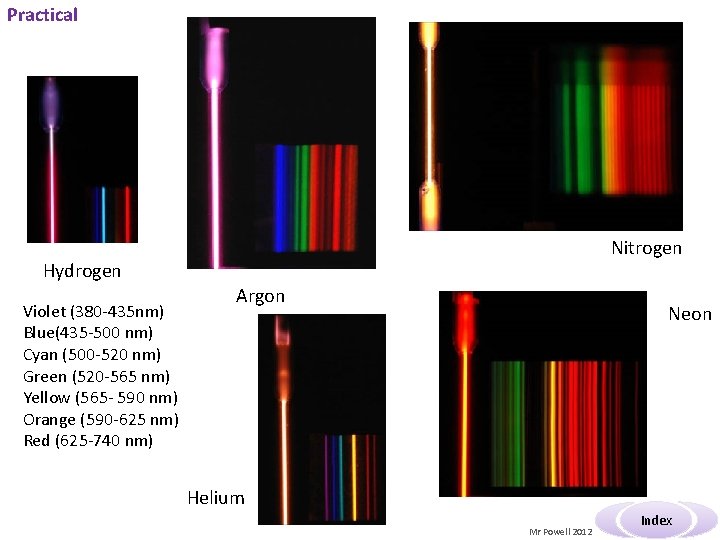
Practical Nitrogen Hydrogen Violet (380 -435 nm) Blue(435 -500 nm) Cyan (500 -520 nm) Green (520 -565 nm) Yellow (565 - 590 nm) Orange (590 -625 nm) Red (625 -740 nm) Argon Neon Helium Mr Powell 2012 Index

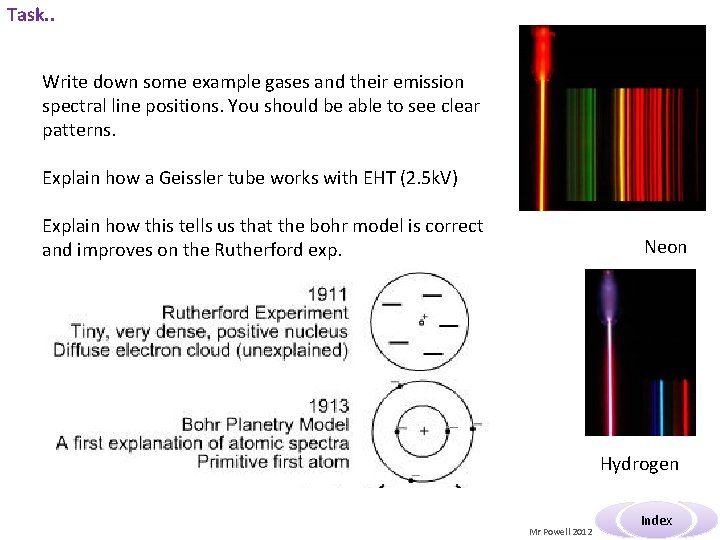
Task. . Write down some example gases and their emission spectral line positions. You should be able to see clear patterns. Explain how a Geissler tube works with EHT (2. 5 k. V) Explain how this tells us that the bohr model is correct and improves on the Rutherford exp. Neon Hydrogen Mr Powell 2012 Index

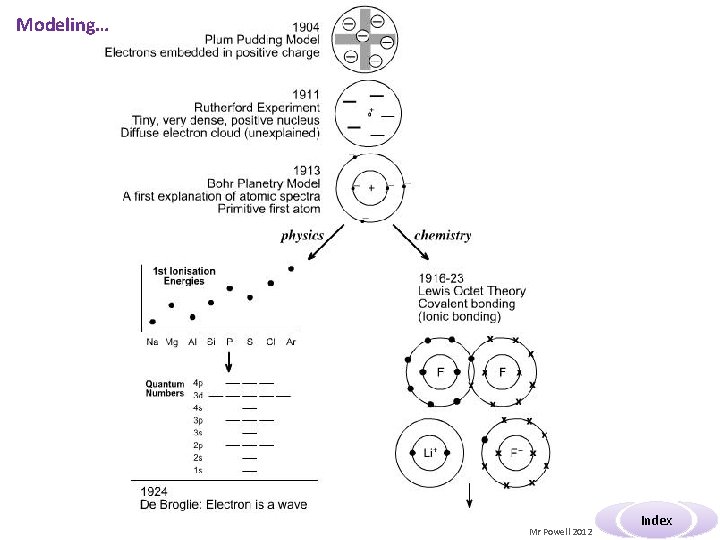
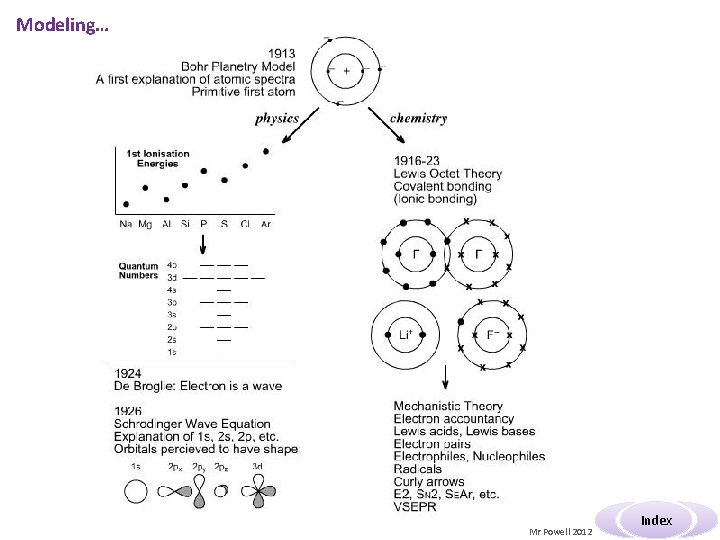
Modeling… Mr Powell 2012 Index

Modeling… Mr Powell 2012 Index

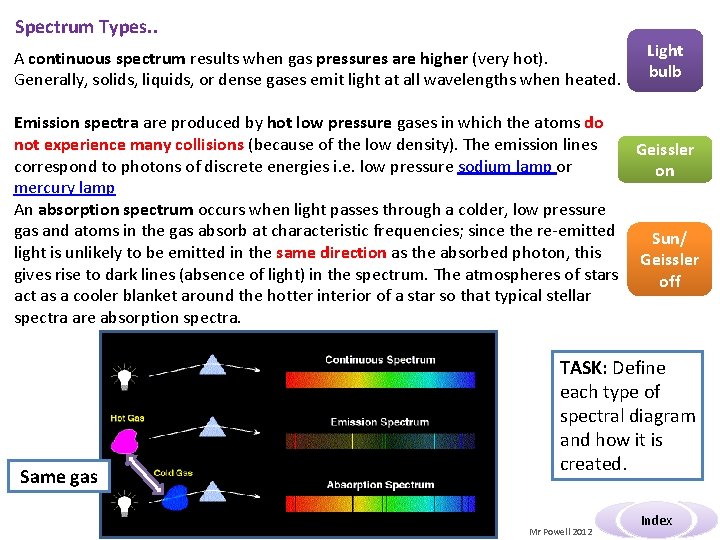
Spectrum Types. . A continuous spectrum results when gas pressures are higher (very hot). Generally, solids, liquids, or dense gases emit light at all wavelengths when heated. Light bulb Emission spectra are produced by hot low pressure gases in which the atoms do not experience many collisions (because of the low density). The emission lines Geissler correspond to photons of discrete energies i. e. low pressure sodium lamp or on mercury lamp An absorption spectrum occurs when light passes through a colder, low pressure gas and atoms in the gas absorb at characteristic frequencies; since the re-emitted Sun/ light is unlikely to be emitted in the same direction as the absorbed photon, this Geissler gives rise to dark lines (absence of light) in the spectrum. The atmospheres of stars off act as a cooler blanket around the hotter interior of a star so that typical stellar spectra are absorption spectra. Same gas TASK: Define each type of spectral diagram and how it is created. Mr Powell 2012 Index

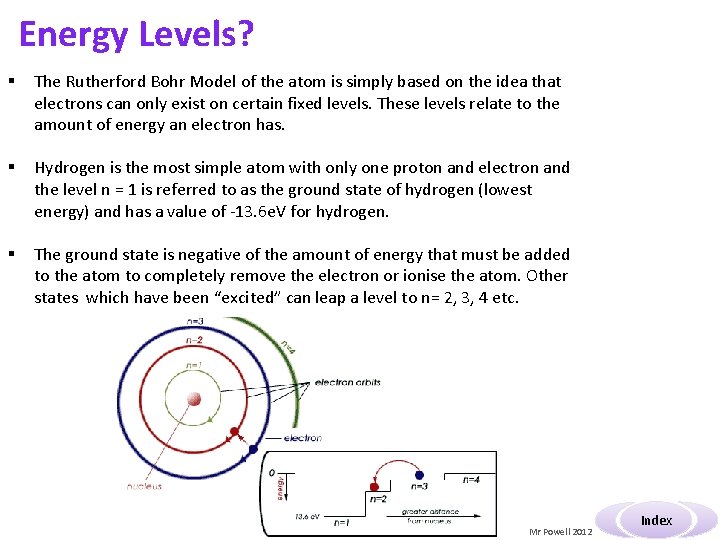
Energy Levels? § The Rutherford Bohr Model of the atom is simply based on the idea that electrons can only exist on certain fixed levels. These levels relate to the amount of energy an electron has. § Hydrogen is the most simple atom with only one proton and electron and the level n = 1 is referred to as the ground state of hydrogen (lowest energy) and has a value of -13. 6 e. V for hydrogen. § The ground state is negative of the amount of energy that must be added to the atom to completely remove the electron or ionise the atom. Other states which have been “excited” can leap a level to n= 2, 3, 4 etc. Mr Powell 2012 Index

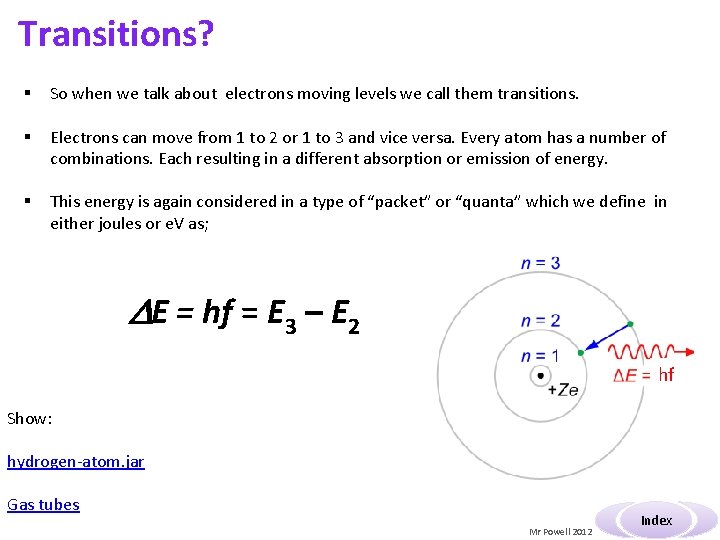
Transitions? § So when we talk about electrons moving levels we call them transitions. § Electrons can move from 1 to 2 or 1 to 3 and vice versa. Every atom has a number of combinations. Each resulting in a different absorption or emission of energy. § This energy is again considered in a type of “packet” or “quanta” which we define in either joules or e. V as; E = hf = E 3 – E 2 hf Show: hydrogen-atom. jar Gas tubes Mr Powell 2012 Index

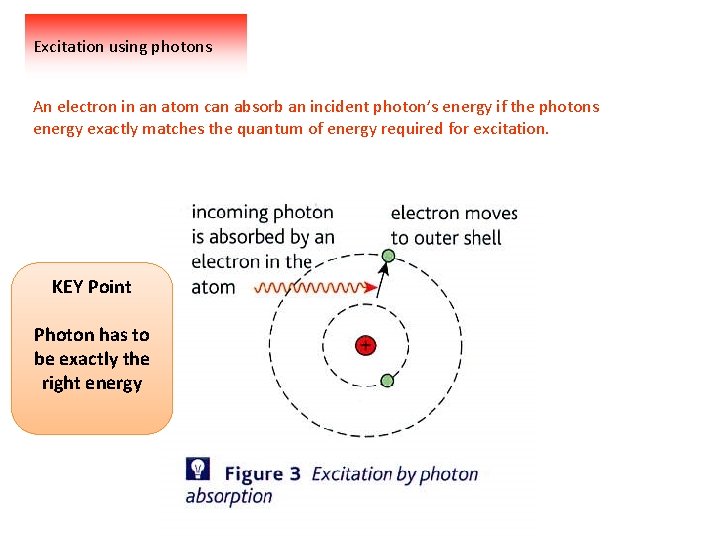
Excitation using photons An electron in an atom can absorb an incident photon’s energy if the photons energy exactly matches the quantum of energy required for excitation. KEY Point Photon has to be exactly the right energy

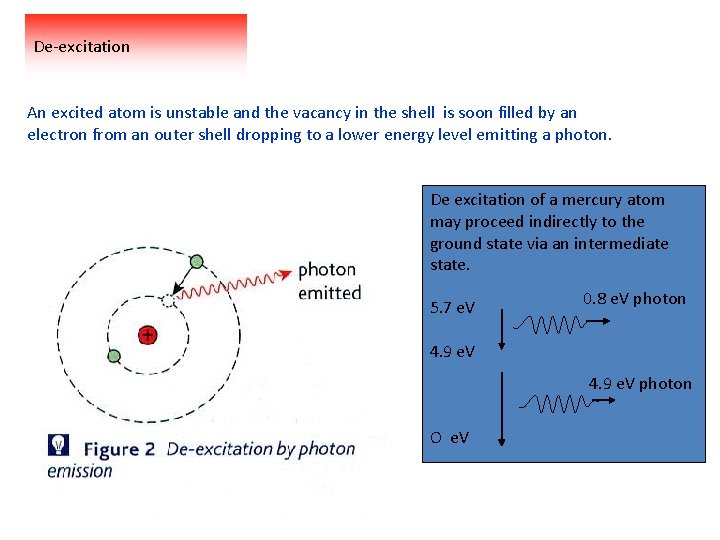
De-excitation An excited atom is unstable and the vacancy in the shell is soon filled by an electron from an outer shell dropping to a lower energy level emitting a photon. De excitation of a mercury atom may proceed indirectly to the ground state via an intermediate state. 5. 7 e. V 0. 8 e. V photon 4. 9 e. V photon O e. V

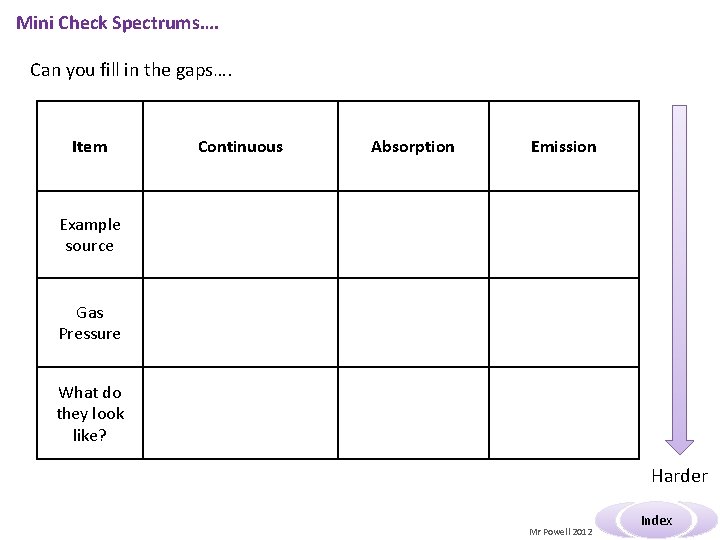
Mini Check Spectrums…. Can you fill in the gaps…. Item Continuous Absorption Emission Example source Gas Pressure What do they look like? Harder Mr Powell 2012 Index

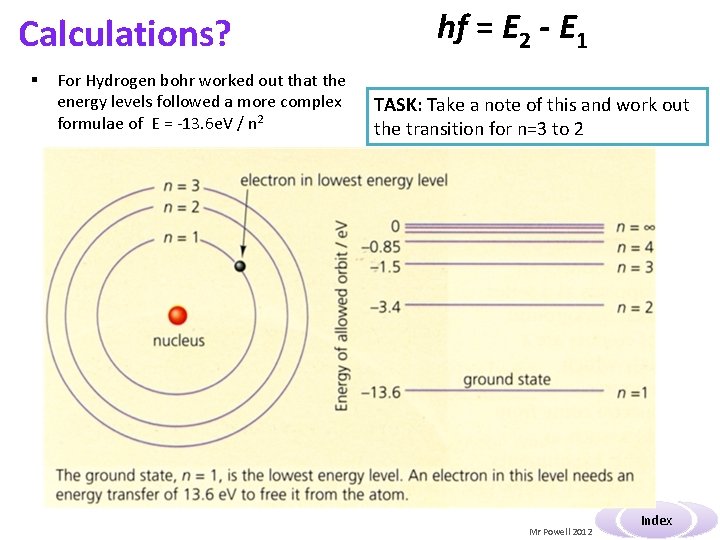
Calculations? § For Hydrogen bohr worked out that the energy levels followed a more complex formulae of E = -13. 6 e. V / n 2 hf = E 2 - E 1 TASK: Take a note of this and work out the transition for n=3 to 2 Mr Powell 2012 Index

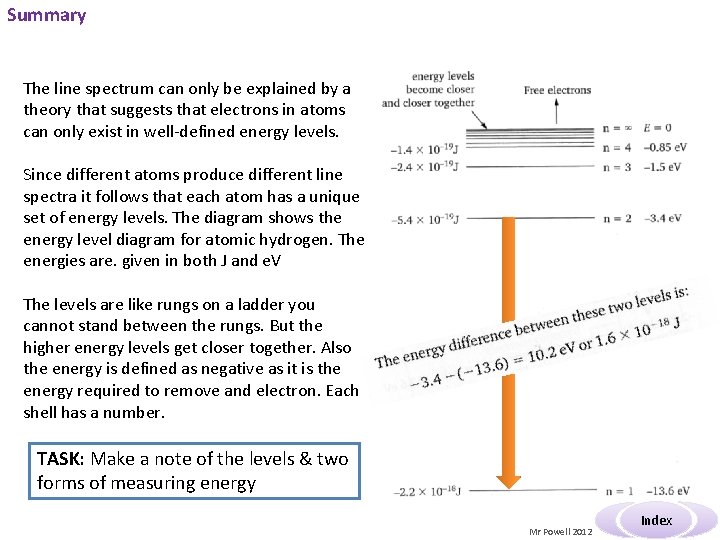
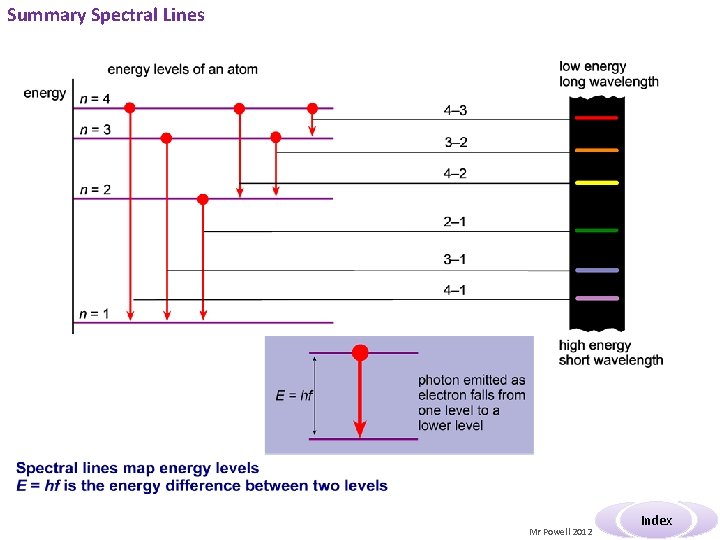
Summary The line spectrum can only be explained by a theory that suggests that electrons in atoms can only exist in well-defined energy levels. Since different atoms produce different line spectra it follows that each atom has a unique set of energy levels. The diagram shows the energy level diagram for atomic hydrogen. The energies are. given in both J and e. V The levels are like rungs on a ladder you cannot stand between the rungs. But the higher energy levels get closer together. Also the energy is defined as negative as it is the energy required to remove and electron. Each shell has a number. TASK: Make a note of the levels & two forms of measuring energy Mr Powell 2012 Index

Summary Spectral Lines Mr Powell 2012 Index

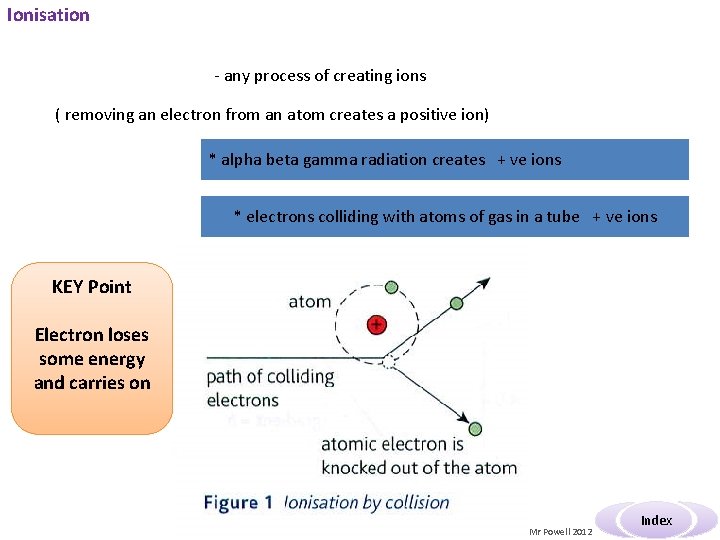
Ionisation - any process of creating ions ( removing an electron from an atom creates a positive ion) * alpha beta gamma radiation creates + ve ions * electrons colliding with atoms of gas in a tube + ve ions KEY Point Electron loses some energy and carries on Mr Powell 2012 Index

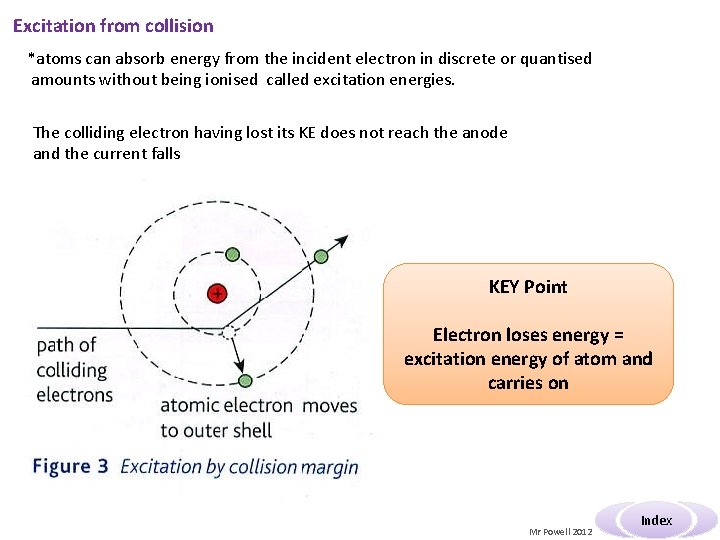
Excitation from collision *atoms can absorb energy from the incident electron in discrete or quantised amounts without being ionised called excitation energies. The colliding electron having lost its KE does not reach the anode and the current falls KEY Point Electron loses energy = excitation energy of atom and carries on Mr Powell 2012 Index

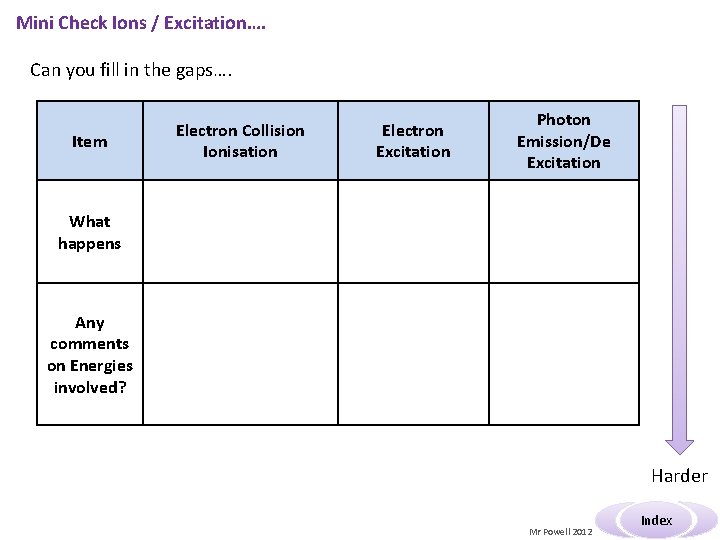
Mini Check Ions / Excitation…. Can you fill in the gaps…. Item Electron Collision Ionisation Electron Excitation Photon Emission/De Excitation What happens Any comments on Energies involved? Harder Mr Powell 2012 Index

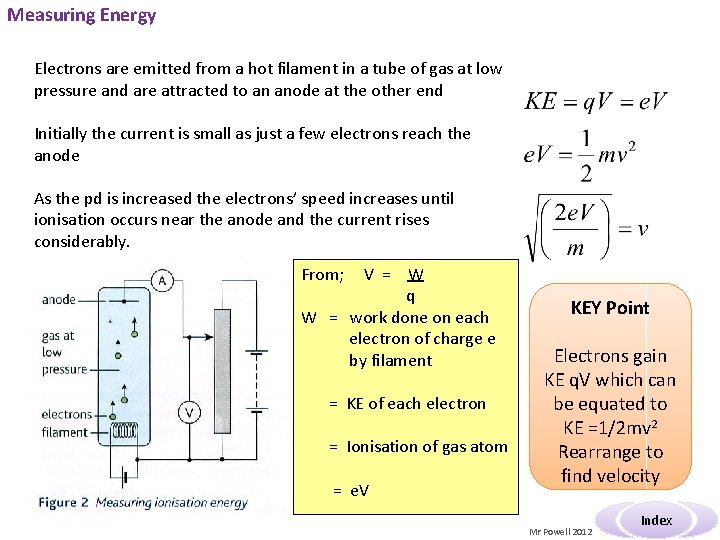
Measuring Energy Electrons are emitted from a hot filament in a tube of gas at low pressure and are attracted to an anode at the other end Initially the current is small as just a few electrons reach the anode As the pd is increased the electrons’ speed increases until ionisation occurs near the anode and the current rises considerably. From; V = W q W = work done on each electron of charge e by filament = KE of each electron = Ionisation of gas atom = e. V KEY Point Electrons gain KE q. V which can be equated to KE =1/2 mv 2 Rearrange to find velocity Mr Powell 2012 Index

Quick think? Using the symbols below can you make any formulae. . h E E 1 c v KEmax λ m p E 2 Φ f Mr Powell 2012 Index

Starter Mr Powell 2012 Index

Exam Question Mr Powell 2012 Index

Connection • • • Connect your learning to the content of the lesson Share the process by which the learning will actually take place Explore the outcomes of the learning, emphasising why this will be beneficial for the learner Demonstration • Use formative feedback – Assessment for Learning • Vary the groupings within the classroom for the purpose of learning – individual; pair; group/team; friendship; teacher selected; single sex; mixed sex • Offer different ways for the students to demonstrate their understanding • Allow the students to “show off” their learning Activation Consolidation • Construct problem-solving challenges for the students • Use a multi-sensory approach – VAK • Promote a language of learning to enable the students to talk about their progress or obstacles to it • Learning as an active process, so the students aren’t passive receptors • Structure active reflection on the lesson content and the process of learning • Seek transfer between “subjects” • Review the learning from this lesson and preview the learning for the next • Promote ways in which the students will remember • A “news broadcast” approach to learning Mr Powell 2012 Index