5 QUANTUM MECHANICS AND ATOMIC STRUCTURE CHAPTER 5


![Radial Distribution Functions: RDFs Prob(r) = r 2[R(r)]2 ┃Ψ┃2 x 4πr 2 dr General Radial Distribution Functions: RDFs Prob(r) = r 2[R(r)]2 ┃Ψ┃2 x 4πr 2 dr General](https://slidetodoc.com/presentation_image/31a83a34f857f6286d3340788435d938/image-21.jpg)

![§ Be, 1 s 22 s 2 or [He]2 s 2 § B, 1 § Be, 1 s 22 s 2 or [He]2 s 2 § B, 1](https://slidetodoc.com/presentation_image/31a83a34f857f6286d3340788435d938/image-46.jpg)
![§ n = 3: Na, [He]2 s 22 p 63 s 1 or [Ne]3 § n = 3: Na, [He]2 s 22 p 63 s 1 or [Ne]3](https://slidetodoc.com/presentation_image/31a83a34f857f6286d3340788435d938/image-50.jpg)


- Slides: 68

5 QUANTUM MECHANICS AND ATOMIC STRUCTURE CHAPTER 5. 1 The Hydrogen Atom 5. 2 Shell Model for Many-Electron Atoms 5. 3 Aufbau Principle and Electron Configurations 5. 4 Shells and the Periodic Table: Photoelectron Spectroscopy 5. 5 Periodic Properties and Electronic Structure General Chemistry I 1

Colors of Fireworks from atomic emission red from Sr orange from Ca yellow from Na green from Ba blue from Cu General Chemistry I 2

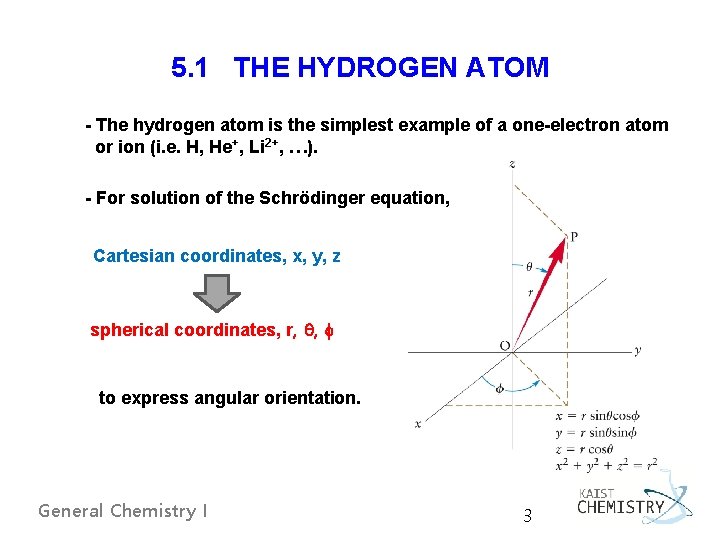
5. 1 THE HYDROGEN ATOM - The hydrogen atom is the simplest example of a one-electron atom or ion (i. e. H, He+, Li 2+, …). - For solution of the Schrödinger equation, Cartesian coordinates, x, y, z spherical coordinates, r, , to express angular orientation. General Chemistry I 3

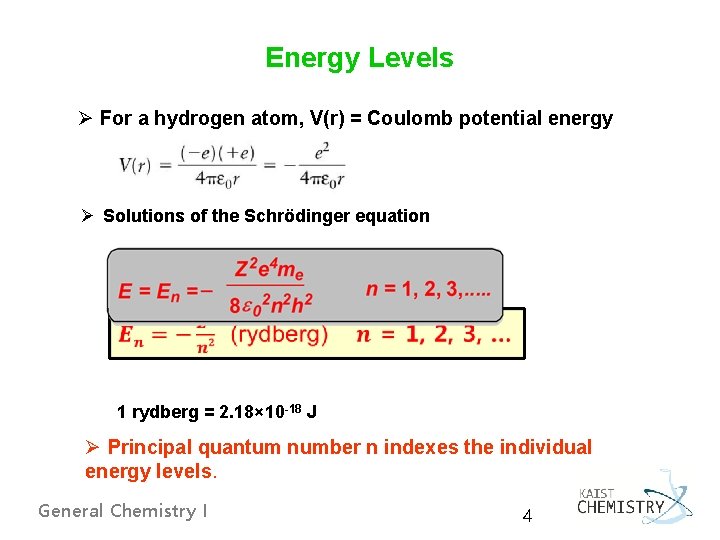
Energy Levels Ø For a hydrogen atom, V(r) = Coulomb potential energy Ø Solutions of the Schrödinger equation 1 rydberg = 2. 18× 10 -18 J Ø Principal quantum number n indexes the individual energy levels. General Chemistry I 4

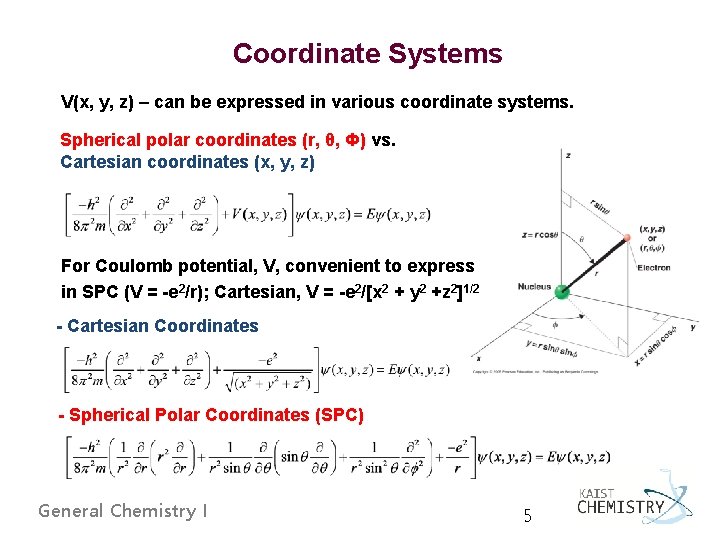
Coordinate Systems V(x, y, z) – can be expressed in various coordinate systems. Spherical polar coordinates (r, θ, Ф) vs. Cartesian coordinates (x, y, z) For Coulomb potential, V, convenient to express in SPC (V = -e 2/r); Cartesian, V = -e 2/[x 2 + y 2 +z 2]1/2 - Cartesian Coordinates - Spherical Polar Coordinates (SPC) General Chemistry I 5

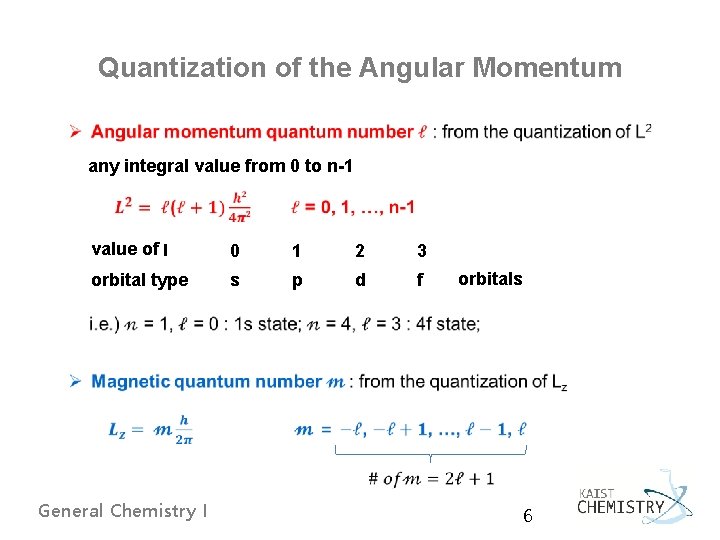
Quantization of the Angular Momentum any integral value from 0 to n-1 value of l 0 1 2 3 orbital type s p d f orbitals General Chemistry I 6

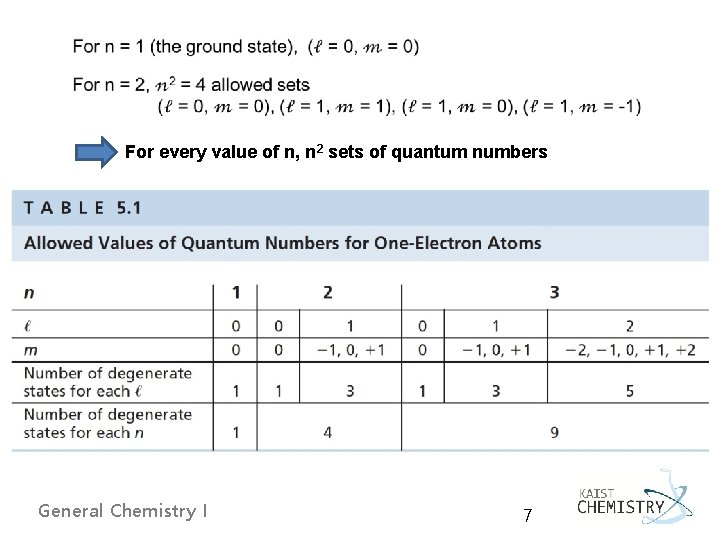
For every value of n, n 2 sets of quantum numbers General Chemistry I 7

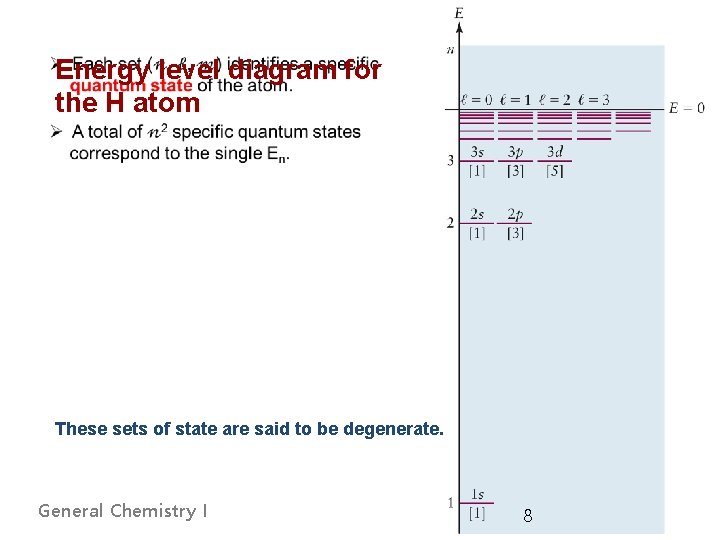
Energy level diagram for the H atom These sets of state are said to be degenerate. General Chemistry I 8

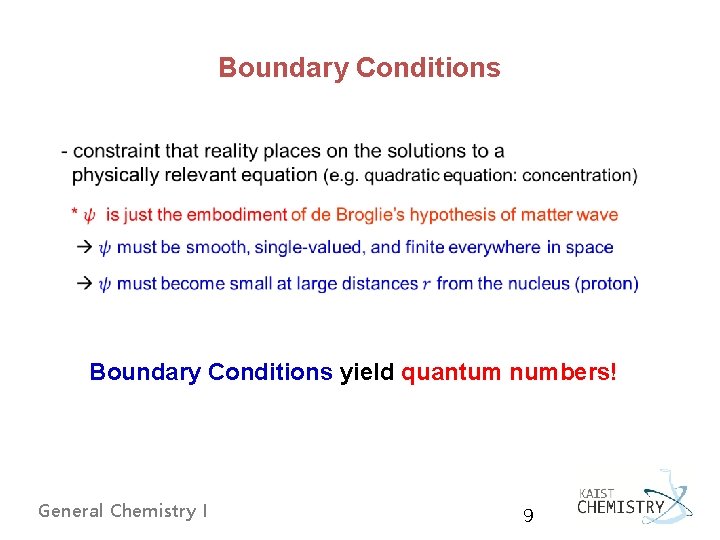
Boundary Conditions yield quantum numbers! General Chemistry I 9

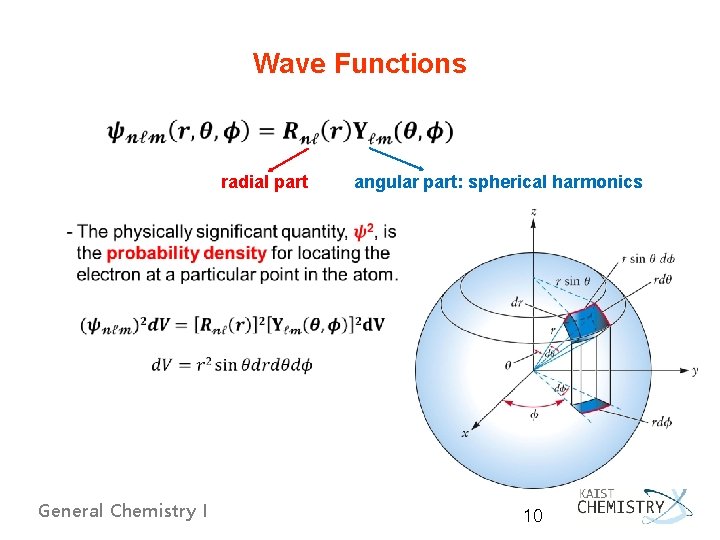
Wave Functions radial part angular part: spherical harmonics General Chemistry I 10

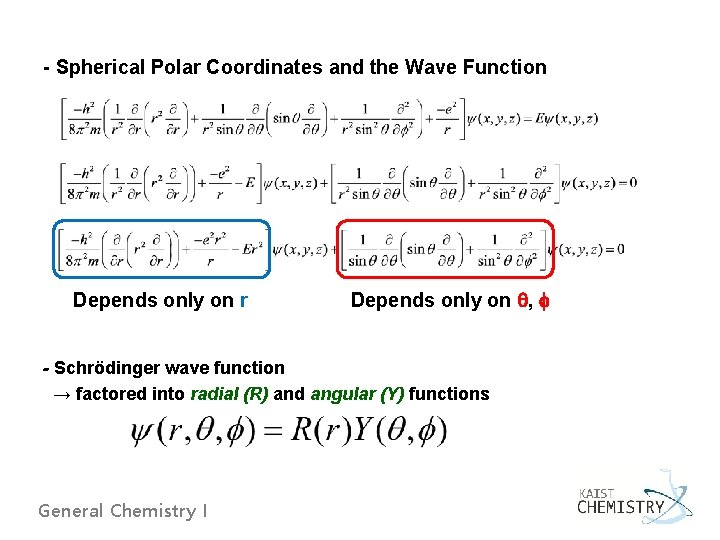
- Spherical Polar Coordinates and the Wave Function Depends only on r Depends only on , - Schrödinger wave function → factored into radial (R) and angular (Y) functions General Chemistry I

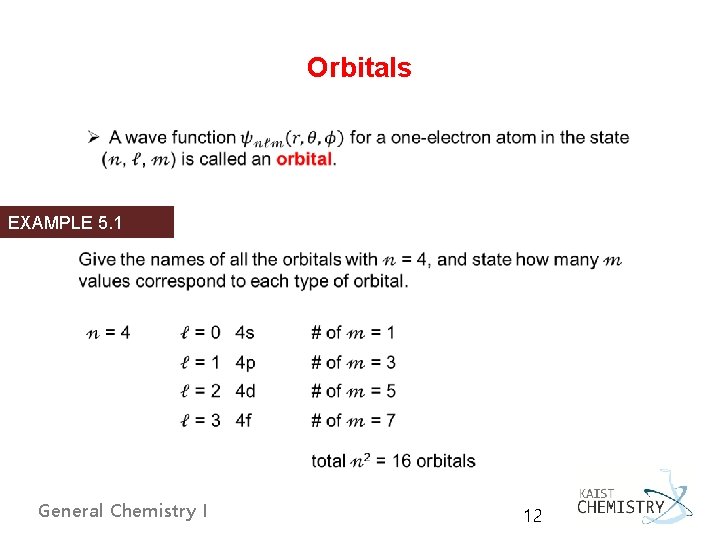
Orbitals EXAMPLE 5. 1 General Chemistry I 12

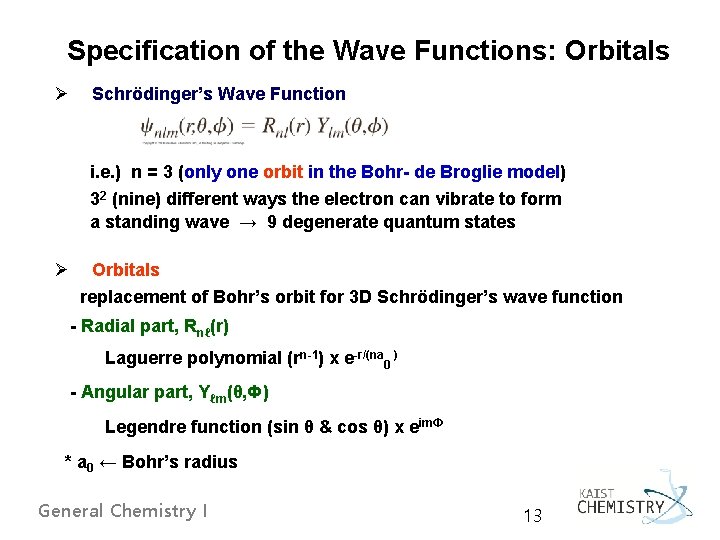
Specification of the Wave Functions: Orbitals Ø Schrödinger’s Wave Function i. e. ) n = 3 (only one orbit in the Bohr- de Broglie model) 32 (nine) different ways the electron can vibrate to form a standing wave → 9 degenerate quantum states Ø Orbitals replacement of Bohr’s orbit for 3 D Schrödinger’s wave function - Radial part, Rnℓ(r) Laguerre polynomial (rn-1) x e-r/(na 0 ) - Angular part, Yℓm(θ, Ф) Legendre function (sin θ & cos θ) x eim. Ф * a 0 ← Bohr’s radius General Chemistry I 13

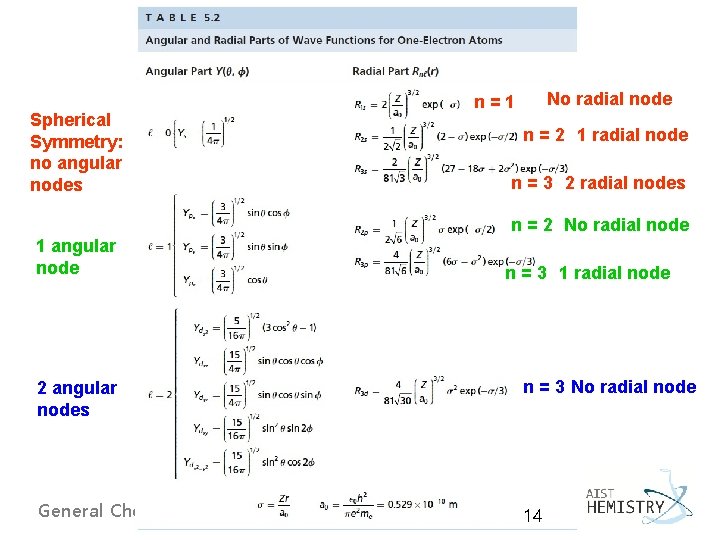
Spherical Symmetry: no angular nodes No radial node n=1 n = 2 1 radial node n = 3 2 radial nodes n = 2 No radial node 1 angular node n = 3 1 radial node 2 angular nodes n = 3 No radial node General Chemistry I 14

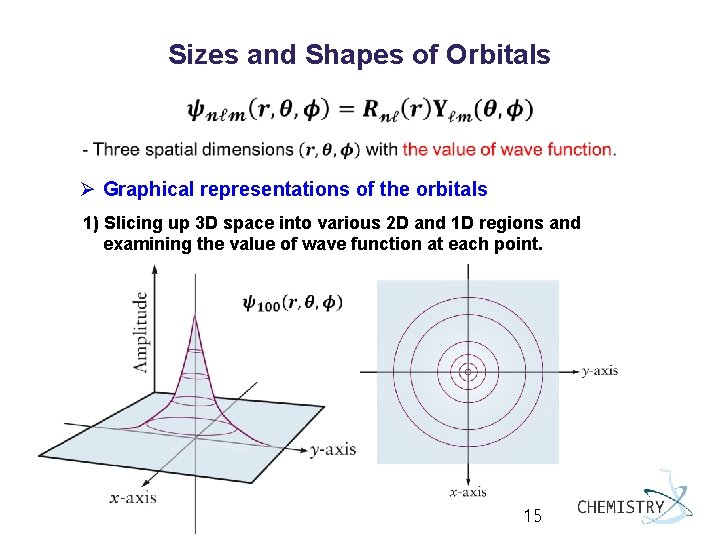
Sizes and Shapes of Orbitals Ø Graphical representations of the orbitals 1) Slicing up 3 D space into various 2 D and 1 D regions and examining the value of wave function at each point. General Chemistry I 15

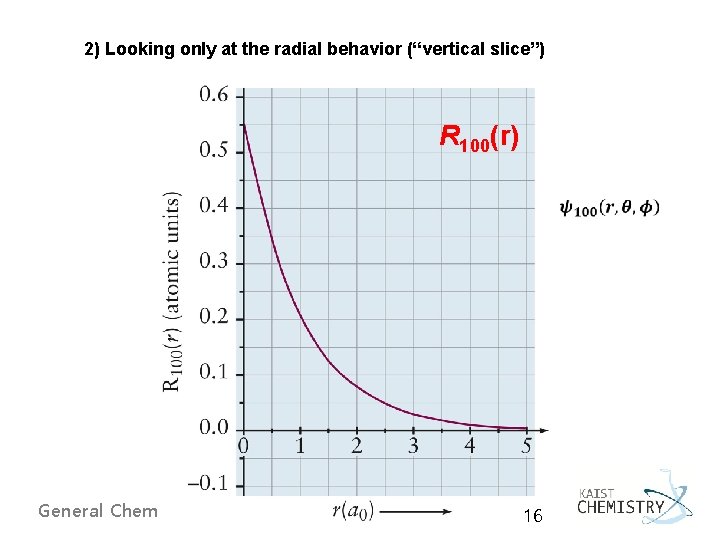
2) Looking only at the radial behavior (“vertical slice”) R 100(r) General Chemistry I 16

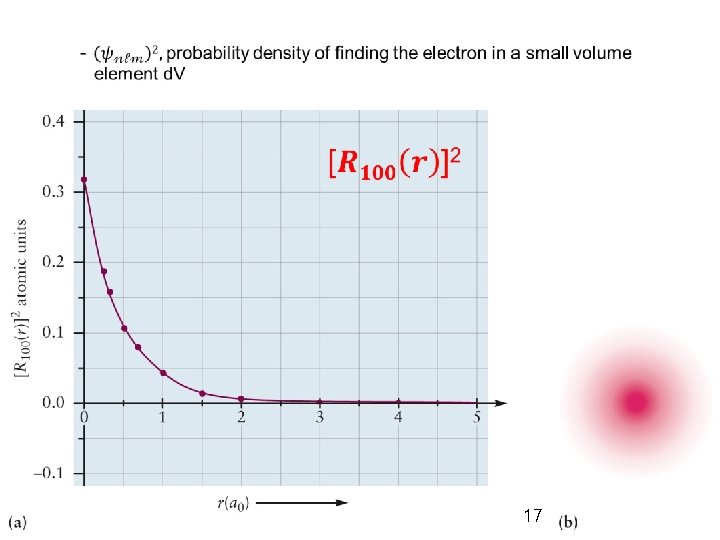
General Chemistry I 17

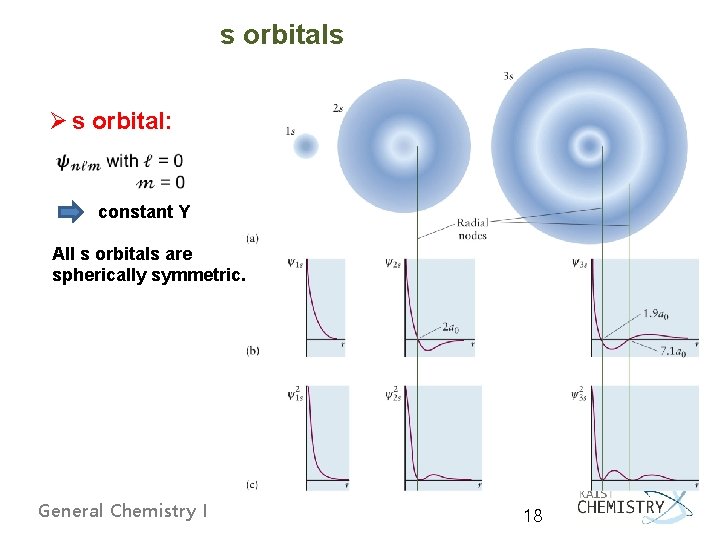
s orbitals Ø s orbital: constant Y All s orbitals are spherically symmetric. General Chemistry I 18

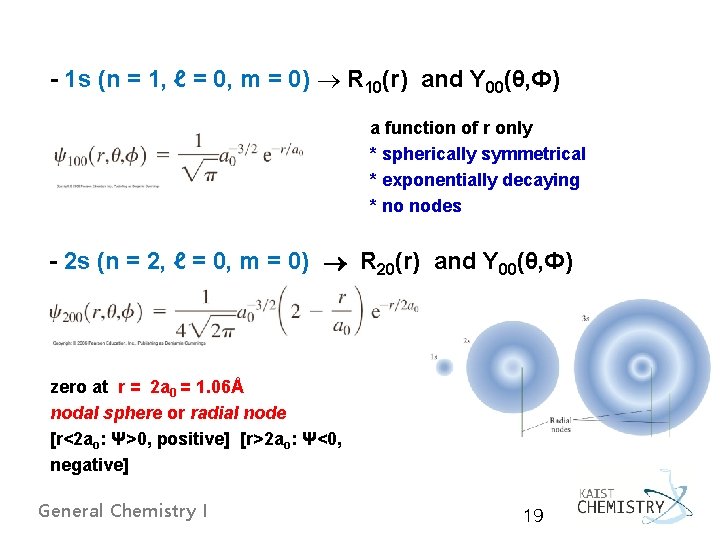
- 1 s (n = 1, ℓ = 0, m = 0) R 10(r) and Y 00(θ, Ф) a function of r only * spherically symmetrical * exponentially decaying * no nodes - 2 s (n = 2, ℓ = 0, m = 0) R 20(r) and Y 00(θ, Ф) zero at r = 2 a 0 = 1. 06Å nodal sphere or radial node [r<2 ao: Ψ>0, positive] [r>2 ao: Ψ<0, negative] General Chemistry I 19

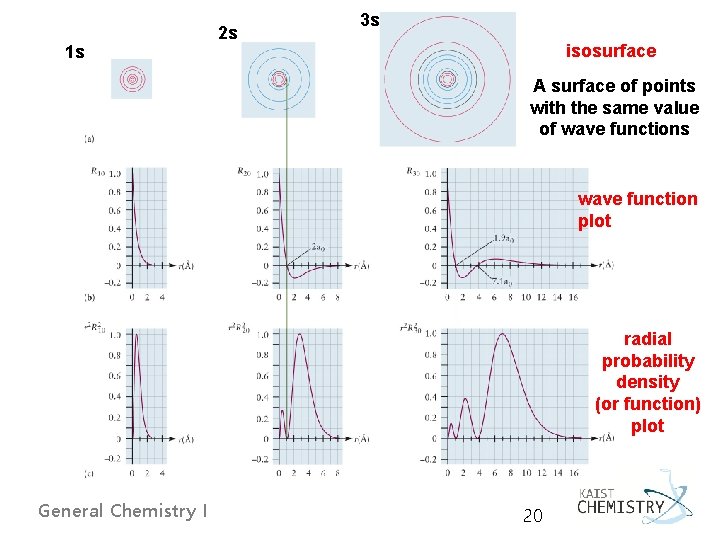
1 s 2 s 3 s isosurface A surface of points with the same value of wave functions wave function plot radial probability density (or function) plot General Chemistry I 20
![Radial Distribution Functions RDFs Probr r 2Rr2 Ψ2 x 4πr 2 dr General Radial Distribution Functions: RDFs Prob(r) = r 2[R(r)]2 ┃Ψ┃2 x 4πr 2 dr General](https://slidetodoc.com/presentation_image/31a83a34f857f6286d3340788435d938/image-21.jpg)
Radial Distribution Functions: RDFs Prob(r) = r 2[R(r)]2 ┃Ψ┃2 x 4πr 2 dr General Chemistry I 21

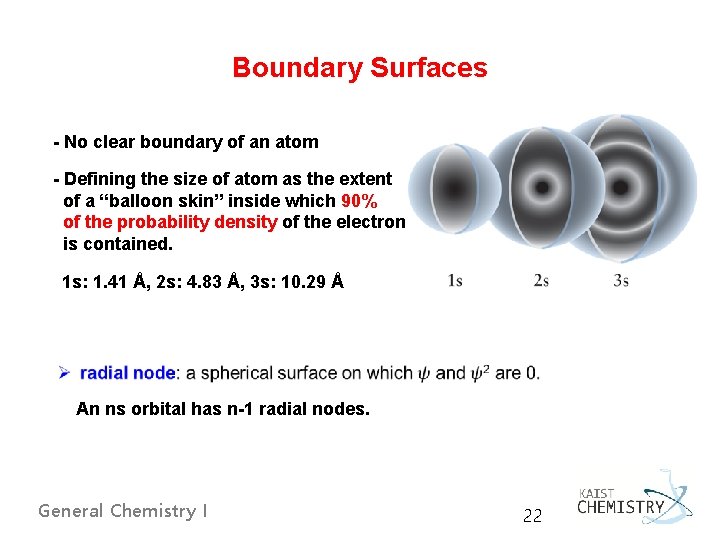
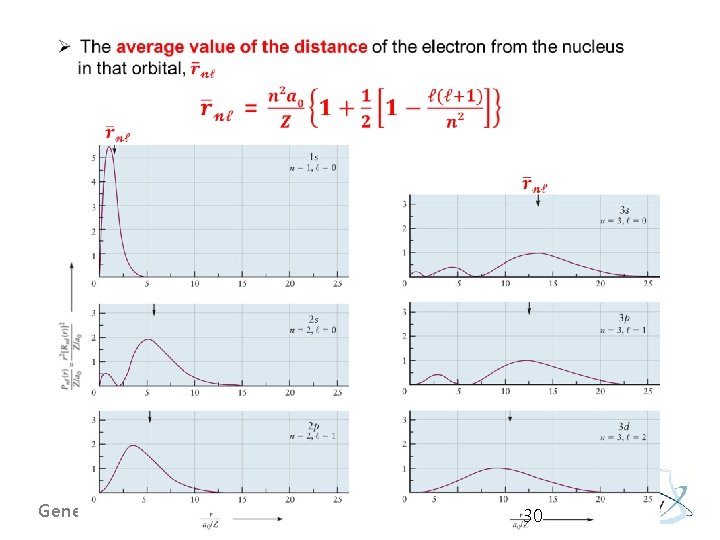
Boundary Surfaces - No clear boundary of an atom - Defining the size of atom as the extent of a “balloon skin” inside which 90% of the probability density of the electron is contained. 1 s: 1. 41 Å, 2 s: 4. 83 Å, 3 s: 10. 29 Å An ns orbital has n-1 radial nodes. General Chemistry I 22

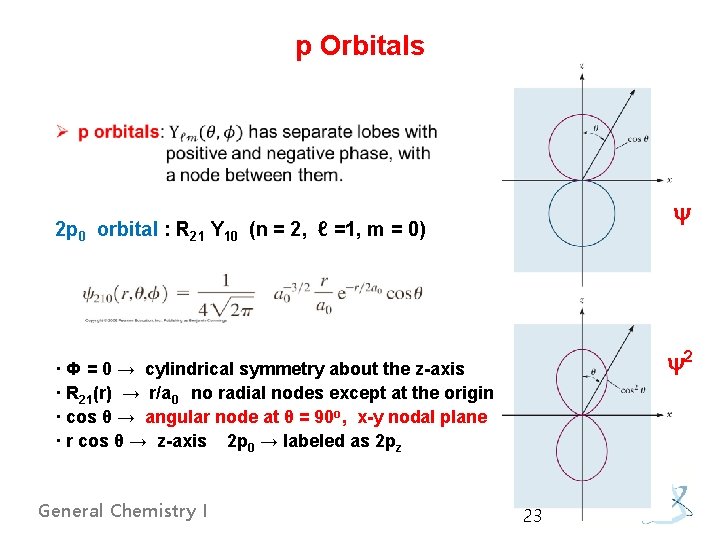
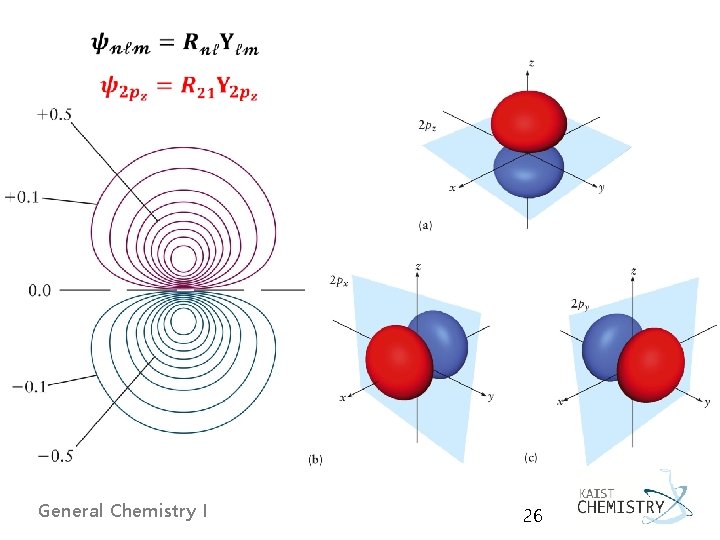
p Orbitals y 2 p 0 orbital : R 21 Y 10 (n = 2, ℓ =1, m = 0) y 2 · Ф = 0 → cylindrical symmetry about the z-axis · R 21(r) → r/a 0 no radial nodes except at the origin · cos θ → angular node at θ = 90 o, x-y nodal plane · r cos θ → z-axis 2 p 0 → labeled as 2 pz General Chemistry I 23

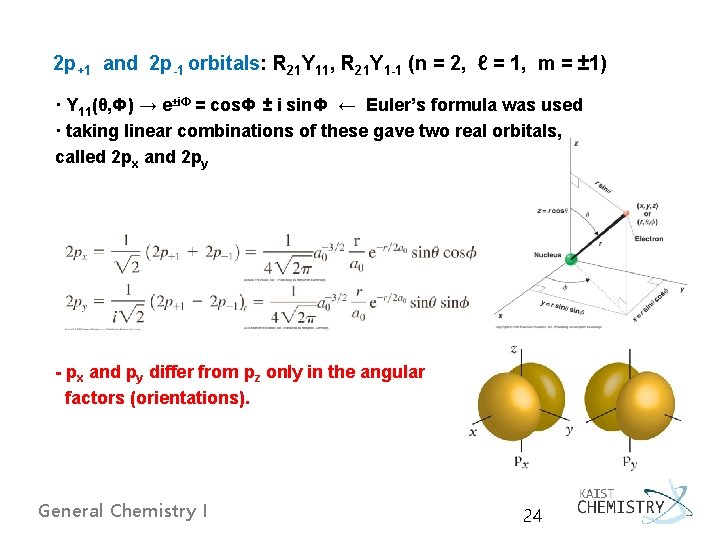
2 p+1 and 2 p-1 orbitals: R 21 Y 11, R 21 Y 1 -1 (n = 2, ℓ = 1, m = ± 1) · Y 11(θ, Ф) → e±i. Ф = cos. Ф ± i sin. Ф ← Euler’s formula was used · taking linear combinations of these gave two real orbitals, called 2 px and 2 py - px and py differ from pz only in the angular factors (orientations). General Chemistry I 24

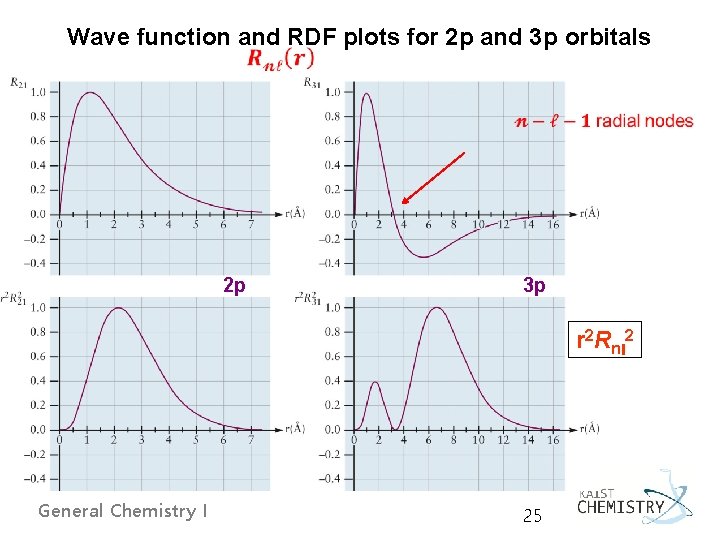
Wave function and RDF plots for 2 p and 3 p orbitals 2 p 3 p r 2 Rnl 2 General Chemistry I 25

General Chemistry I 26

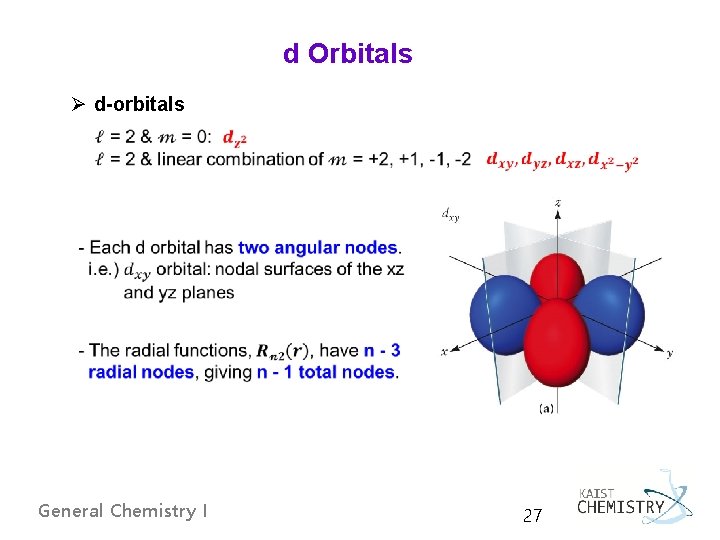
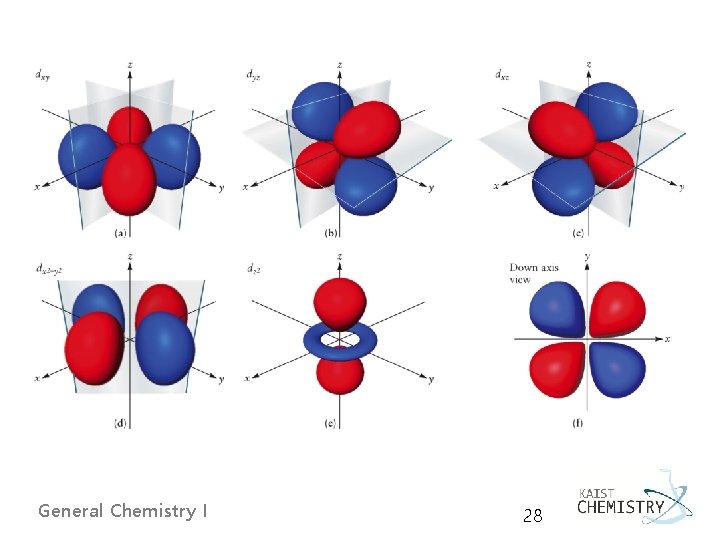
d Orbitals Ø d-orbitals General Chemistry I 27

General Chemistry I 28

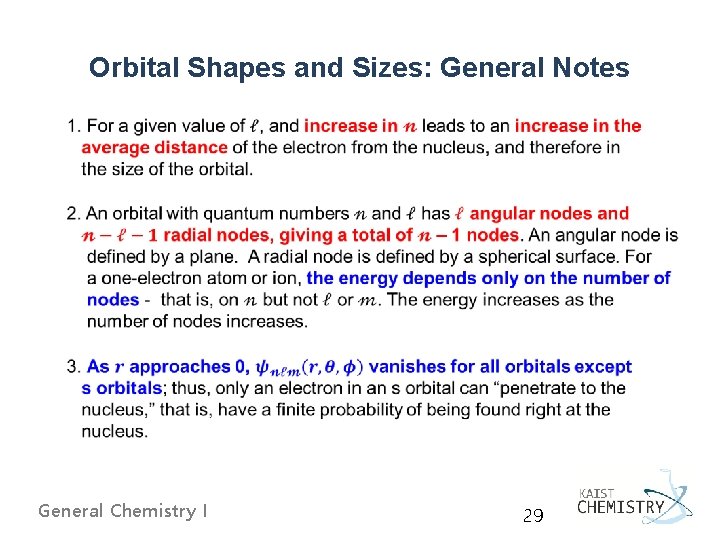
Orbital Shapes and Sizes: General Notes General Chemistry I 29

General Chemistry I 30

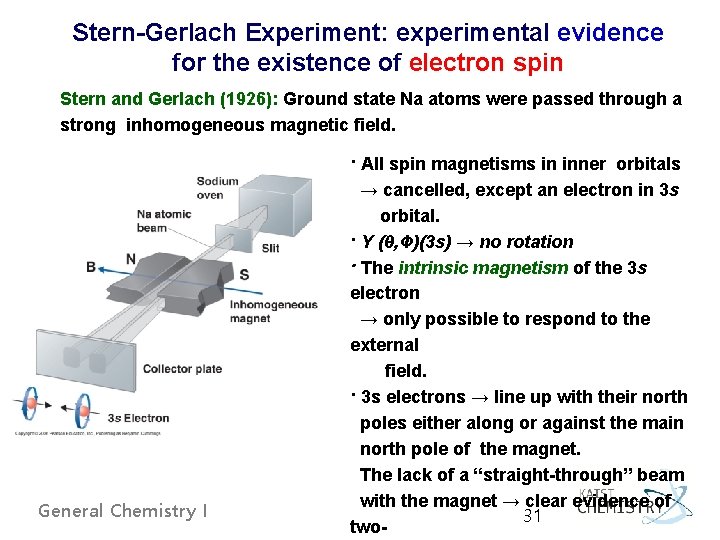
Stern-Gerlach Experiment: experimental evidence for the existence of electron spin Stern and Gerlach (1926): Ground state Na atoms were passed through a strong inhomogeneous magnetic field. General Chemistry I · All spin magnetisms in inner orbitals → cancelled, except an electron in 3 s orbital. · Y (θ, Ф)(3 s) → no rotation · The intrinsic magnetism of the 3 s electron → only possible to respond to the external field. · 3 s electrons → line up with their north poles either along or against the main north pole of the magnet. The lack of a “straight-through” beam with the magnet → clear evidence of 31 two-

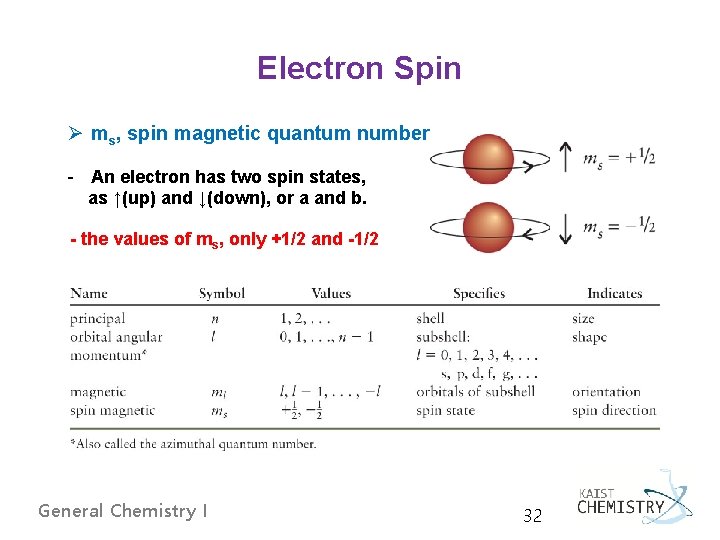
Electron Spin Ø ms, spin magnetic quantum number - An electron has two spin states, as ↑(up) and ↓(down), or a and b. - the values of ms, only +1/2 and -1/2 General Chemistry I 32

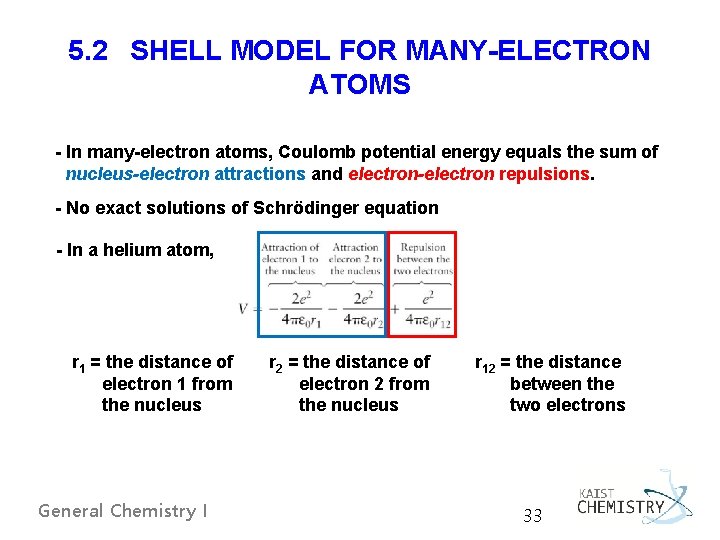
5. 2 SHELL MODEL FOR MANY-ELECTRON ATOMS - In many-electron atoms, Coulomb potential energy equals the sum of nucleus-electron attractions and electron-electron repulsions. - No exact solutions of Schrödinger equation - In a helium atom, r 1 = the distance of electron 1 from the nucleus General Chemistry I r 2 = the distance of electron 2 from the nucleus r 12 = the distance between the two electrons 33

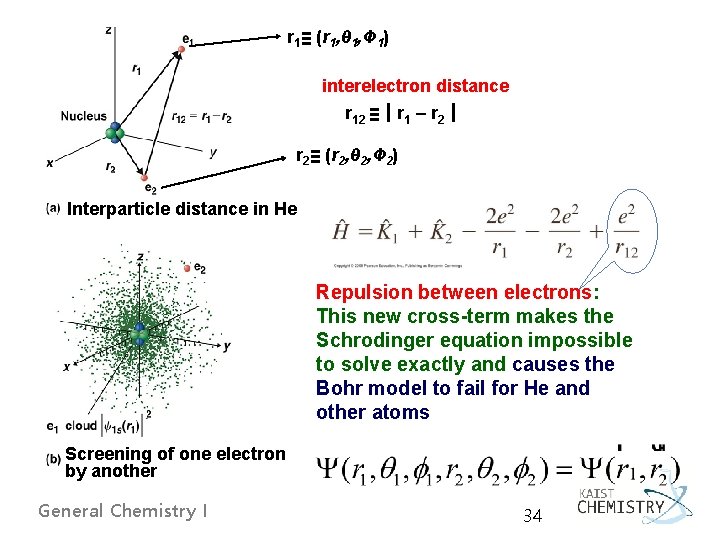
r 1≡ (r 1, θ 1, Ф 1) interelectron distance r 12 ≡┃r 1 – r 2┃ r 2≡ (r 2, θ 2, Ф 2) Interparticle distance in He Repulsion between electrons: This new cross-term makes the Schrodinger equation impossible to solve exactly and causes the Bohr model to fail for He and other atoms Screening of one electron by another General Chemistry I 34

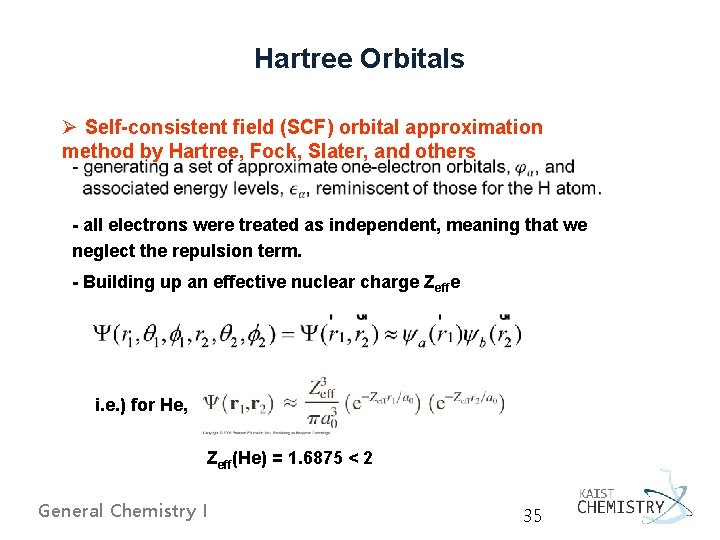
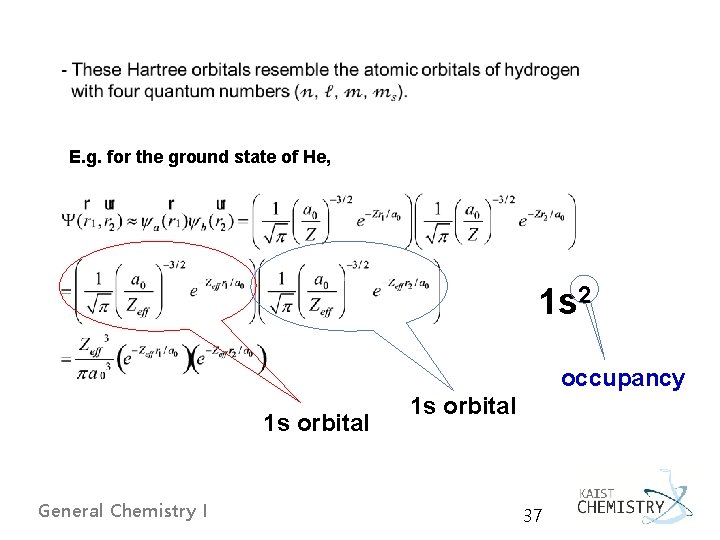
Hartree Orbitals Ø Self-consistent field (SCF) orbital approximation method by Hartree, Fock, Slater, and others - all electrons were treated as independent, meaning that we neglect the repulsion term. - Building up an effective nuclear charge Zeffe i. e. ) for He, Zeff(He) = 1. 6875 < 2 General Chemistry I 35

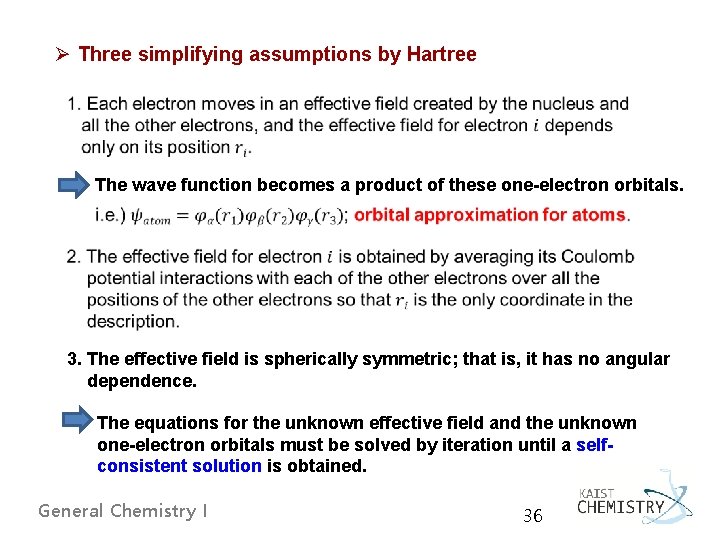
Ø Three simplifying assumptions by Hartree The wave function becomes a product of these one-electron orbitals. 3. The effective field is spherically symmetric; that is, it has no angular dependence. The equations for the unknown effective field and the unknown one-electron orbitals must be solved by iteration until a selfconsistent solution is obtained. General Chemistry I 36

E. g. for the ground state of He, 1 s 2 occupancy 1 s orbital General Chemistry I 1 s orbital 37

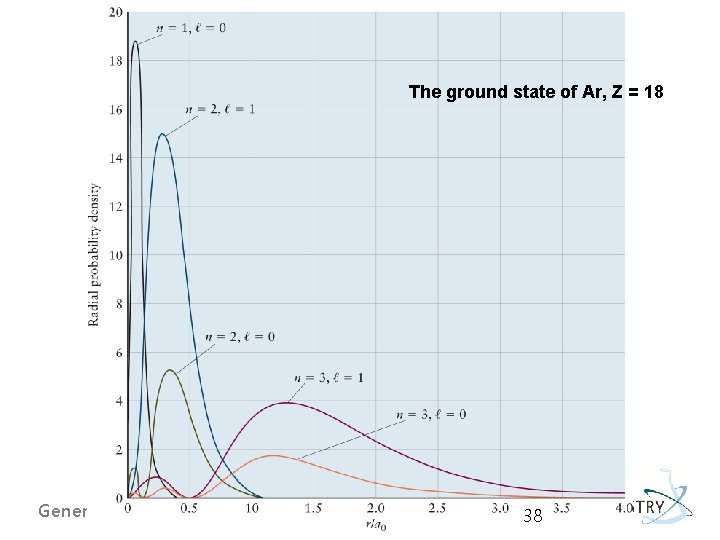
The ground state of Ar, Z = 18 General Chemistry I 38

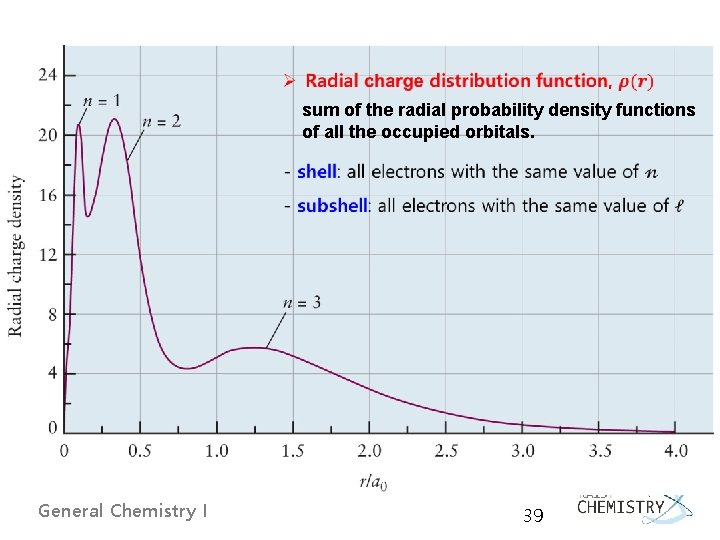
sum of the radial probability density functions of all the occupied orbitals. General Chemistry I 39

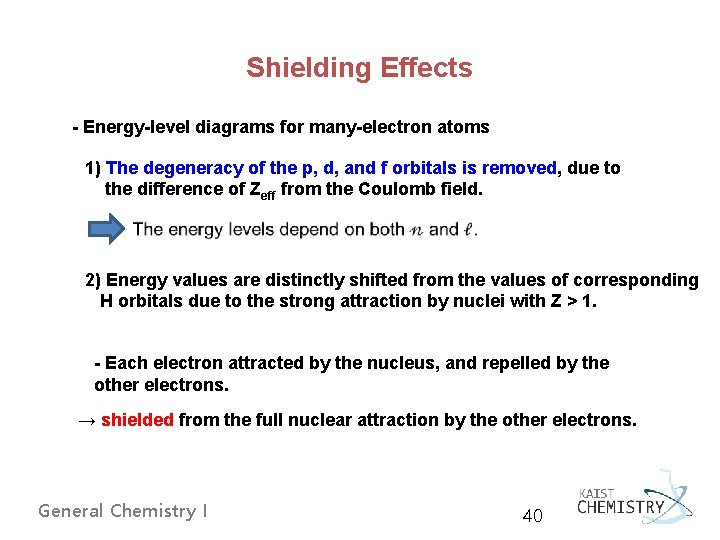
Shielding Effects - Energy-level diagrams for many-electron atoms 1) The degeneracy of the p, d, and f orbitals is removed, due to the difference of Zeff from the Coulomb field. 2) Energy values are distinctly shifted from the values of corresponding H orbitals due to the strong attraction by nuclei with Z > 1. - Each electron attracted by the nucleus, and repelled by the other electrons. → shielded from the full nuclear attraction by the other electrons. General Chemistry I 40

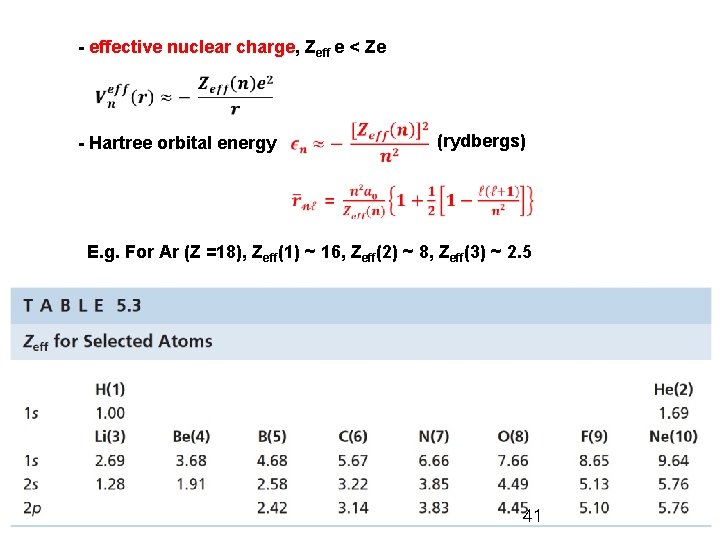
- effective nuclear charge, Zeff e < Ze - Hartree orbital energy (rydbergs) E. g. For Ar (Z =18), Zeff(1) ~ 16, Zeff(2) ~ 8, Zeff(3) ~ 2. 5 General Chemistry I 41

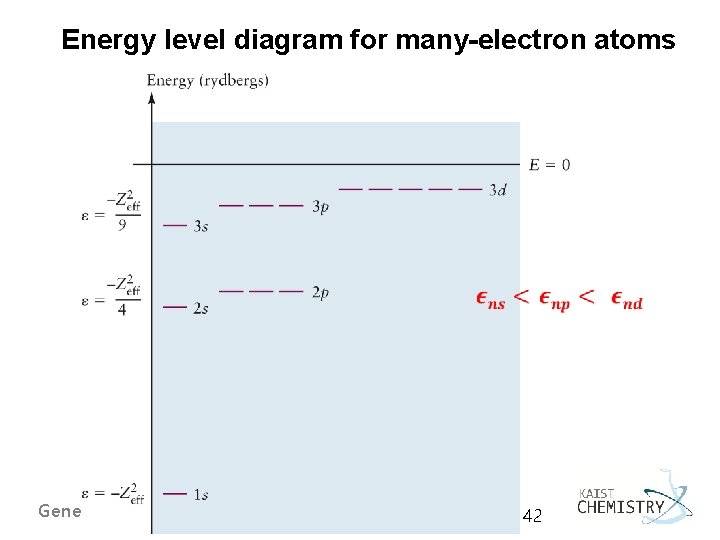
Energy level diagram for many-electron atoms General Chemistry I 42

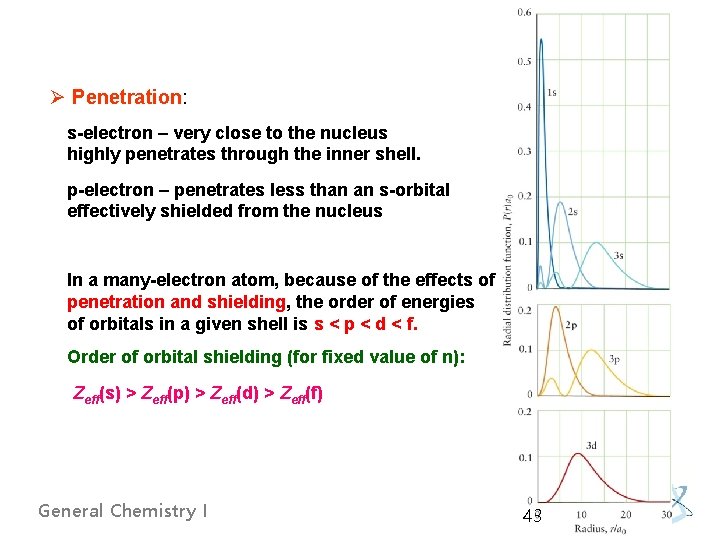
Ø Penetration: s-electron – very close to the nucleus highly penetrates through the inner shell. p-electron – penetrates less than an s-orbital effectively shielded from the nucleus In a many-electron atom, because of the effects of penetration and shielding, the order of energies of orbitals in a given shell is s < p < d < f. Order of orbital shielding (for fixed value of n): Zeff(s) > Zeff(p) > Zeff(d) > Zeff(f) General Chemistry I 43

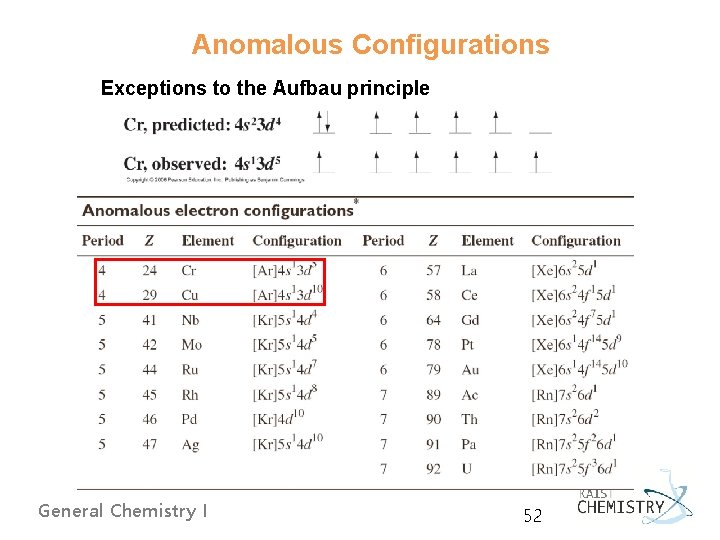
5. 3 AUFBAU PRINCIPLE AND ELECTRON CONFIGURATIONS The Building-up (Aufbau) Principle is a set of rules that allows us to construct ground state electron configurations of the elements. 1. 2. 3. Assume electrons ‘occupy’ orbitals in such a way as to minimize the total energy (lowest energy first). Assume a maximum of two electrons can ‘occupy’ an orbital and these must have opposite spins. (Pauli’s Exclusion Principle: no two electrons in an atom can have the same set of quantum numbers). Assume electrons occupy unoccupied degenerate subshell orbitals first, and with parallel spins (Hund’s rule). In building-up electron configurations, in order of energy, subshell energy overlap must be taken into account (see later). General Chemistry I 44

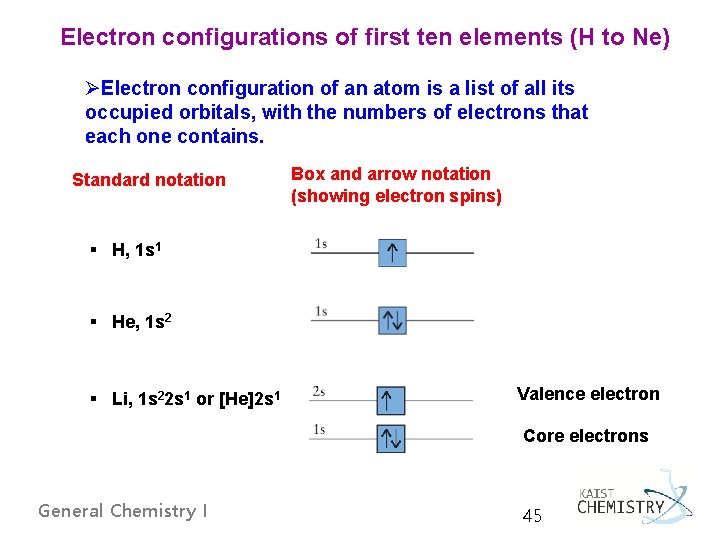
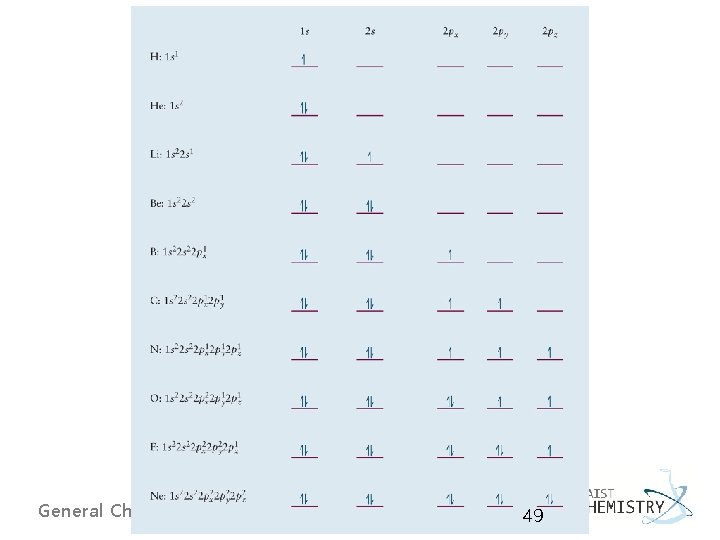
Electron configurations of first ten elements (H to Ne) ØElectron configuration of an atom is a list of all its occupied orbitals, with the numbers of electrons that each one contains. Standard notation Box and arrow notation (showing electron spins) § H, 1 s 1 § He, 1 s 2 § Li, 1 s 22 s 1 or [He]2 s 1 Valence electron Core electrons General Chemistry I 45
![Be 1 s 22 s 2 or He2 s 2 B 1 § Be, 1 s 22 s 2 or [He]2 s 2 § B, 1](https://slidetodoc.com/presentation_image/31a83a34f857f6286d3340788435d938/image-46.jpg)
§ Be, 1 s 22 s 2 or [He]2 s 2 § B, 1 s 22 p 1 or [He]2 s 22 p 1 § C, 1 s 22 p 2 or [He]2 s 22 p 2 → 1 s 22 px 12 py 1 General Chemistry I parallel spins by Hund’s rule 46

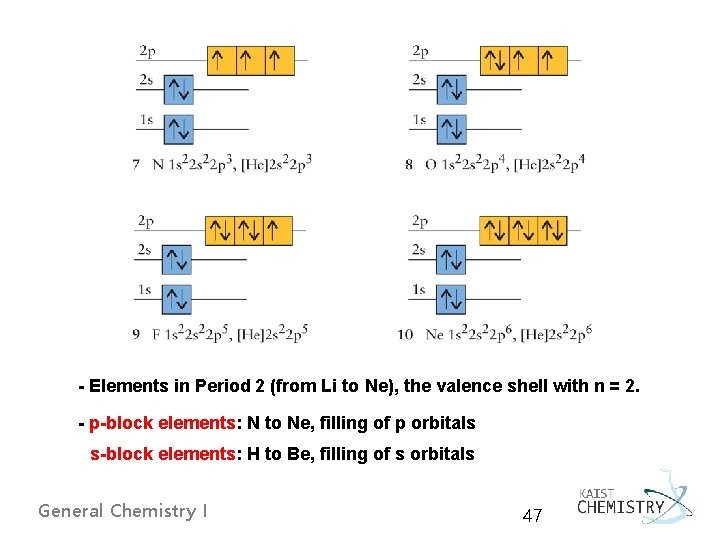
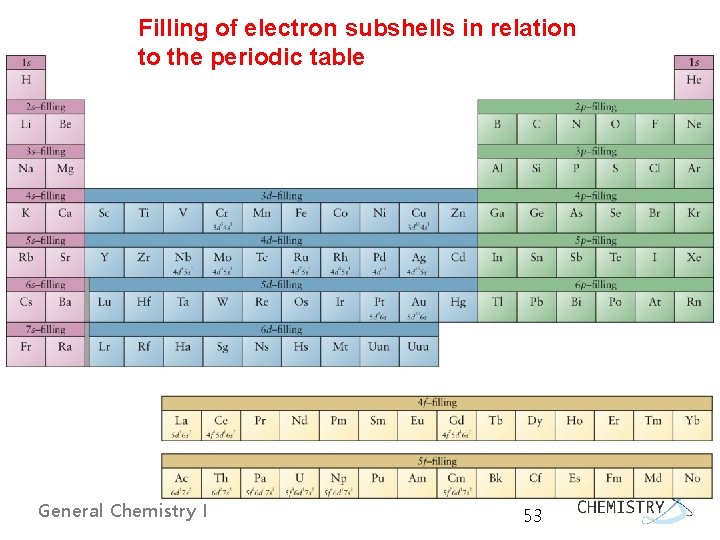
- Elements in Period 2 (from Li to Ne), the valence shell with n = 2. - p-block elements: N to Ne, filling of p orbitals s-block elements: H to Be, filling of s orbitals General Chemistry I 47

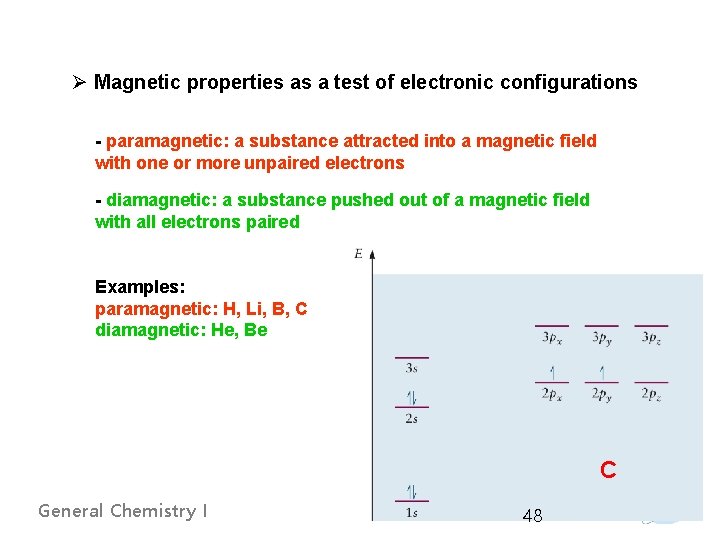
Ø Magnetic properties as a test of electronic configurations - paramagnetic: a substance attracted into a magnetic field with one or more unpaired electrons - diamagnetic: a substance pushed out of a magnetic field with all electrons paired Examples: paramagnetic: H, Li, B, C diamagnetic: He, Be C General Chemistry I 48

General Chemistry I 49
![n 3 Na He2 s 22 p 63 s 1 or Ne3 § n = 3: Na, [He]2 s 22 p 63 s 1 or [Ne]3](https://slidetodoc.com/presentation_image/31a83a34f857f6286d3340788435d938/image-50.jpg)
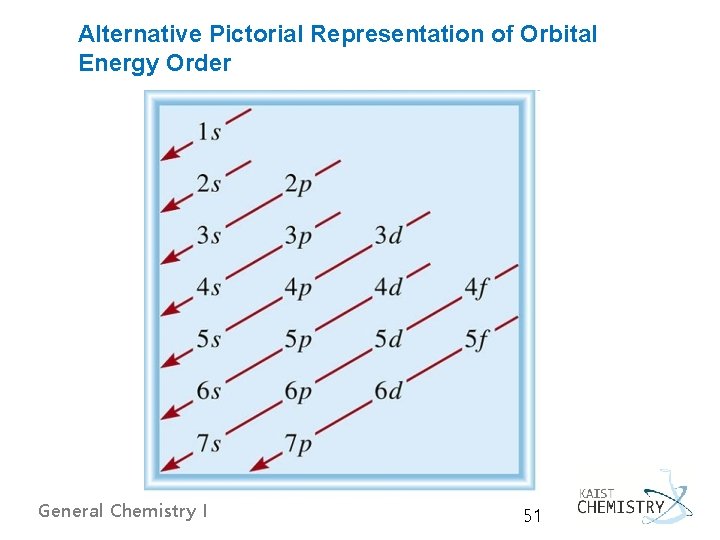
§ n = 3: Na, [He]2 s 22 p 63 s 1 or [Ne]3 s 1 to Ar, [Ne]3 s 23 p 6 § n = 4: from Sc (scandium, Z = 21) to Zn (zinc, Z = 30) the next 10 electrons enter the 3 d-orbitals (d-block elements) Ø The (n+l) rule Order of filling subshells in neutral atoms is determined by filling those with the lowest values of (n+l) first. Subshells in a group with the same value of (n+l) are filled in order of increasing n, due to orbital screening. order: 1 s < 2 p < 3 s < 3 p < 4 s < 3 d < 4 p < 5 s < 4 d < … § n = 5: 5 s-electrons followed by the 4 delectrons n = 6: Ce (cerium, [Xe]4 f 15 d 16 s 2) General Chemistry I 50

Alternative Pictorial Representation of Orbital Energy Order General Chemistry I 51

Anomalous Configurations Exceptions to the Aufbau principle General Chemistry I 52

Filling of electron subshells in relation to the periodic table General Chemistry I 53

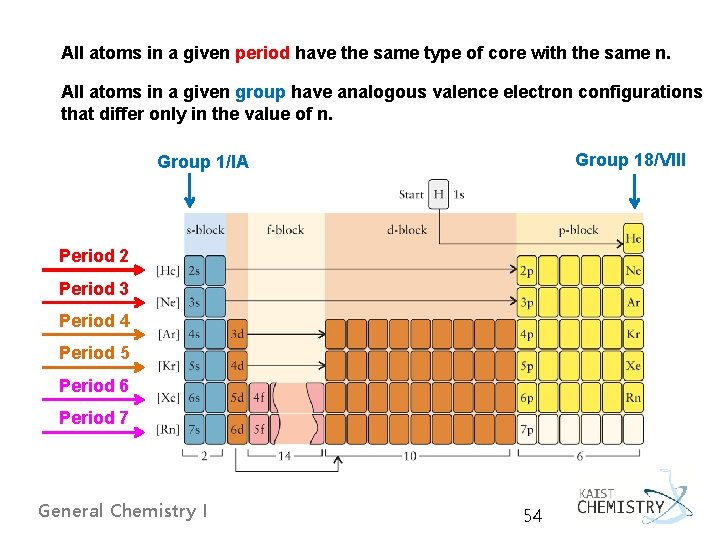
All atoms in a given period have the same type of core with the same n. All atoms in a given group have analogous valence electron configurations that differ only in the value of n. Group 18/VIII Group 1/IA Period 2 Period 3 Period 4 Period 5 Period 6 Period 7 General Chemistry I 54

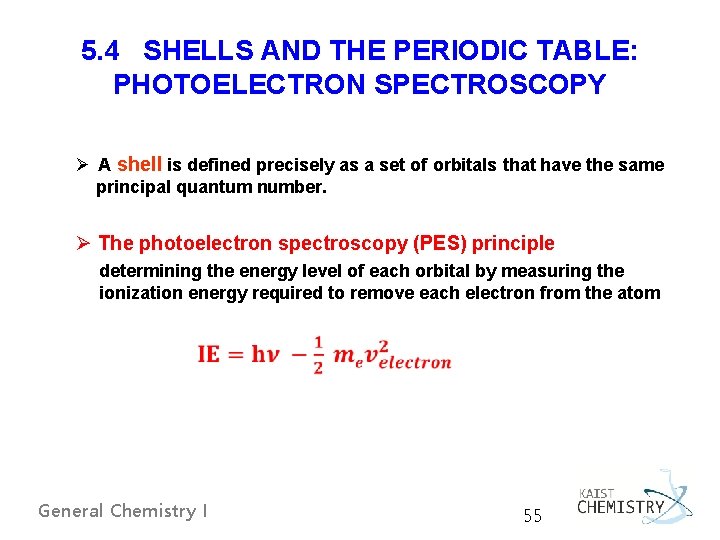
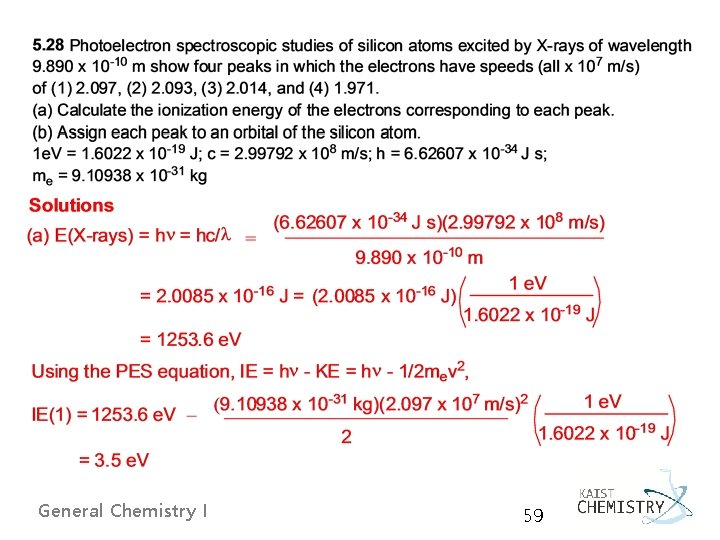
5. 4 SHELLS AND THE PERIODIC TABLE: PHOTOELECTRON SPECTROSCOPY Ø A shell is defined precisely as a set of orbitals that have the same principal quantum number. Ø The photoelectron spectroscopy (PES) principle determining the energy level of each orbital by measuring the ionization energy required to remove each electron from the atom General Chemistry I 55

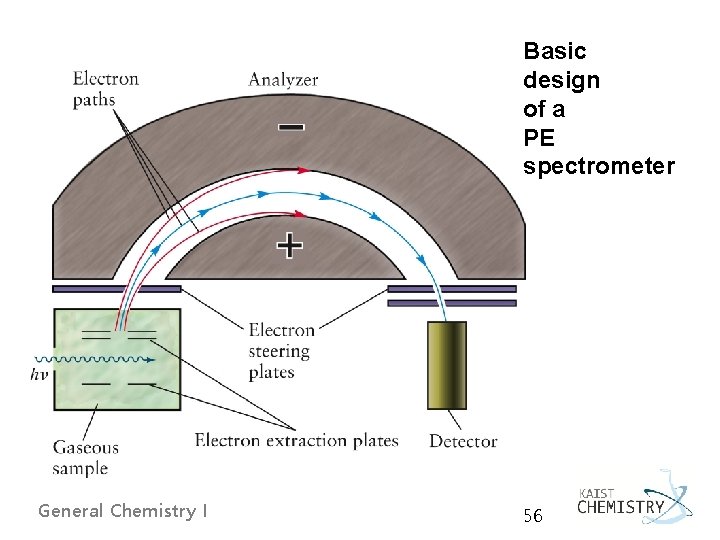
Basic design of a PE spectrometer General Chemistry I 56

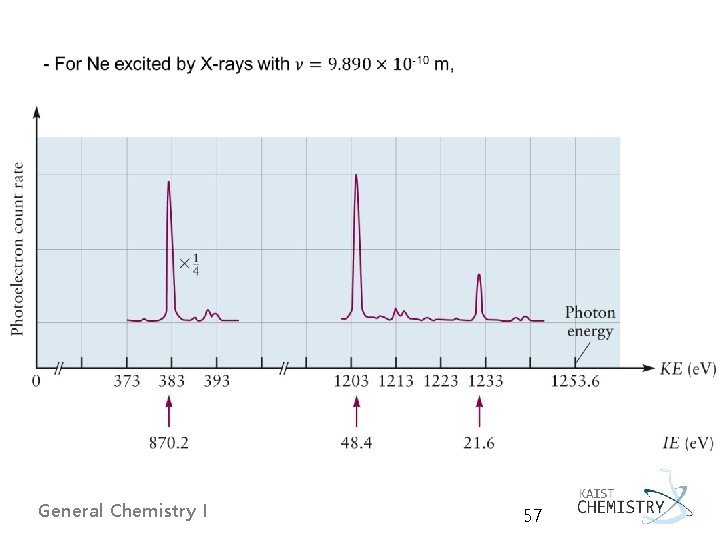
General Chemistry I 57

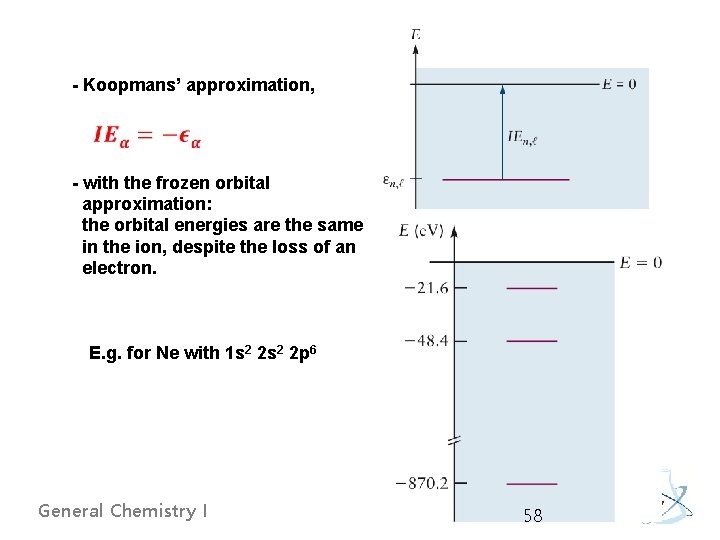
- Koopmans’ approximation, - with the frozen orbital approximation: the orbital energies are the same in the ion, despite the loss of an electron. E. g. for Ne with 1 s 2 2 p 6 General Chemistry I 58

General Chemistry I 59

General Chemistry I 60

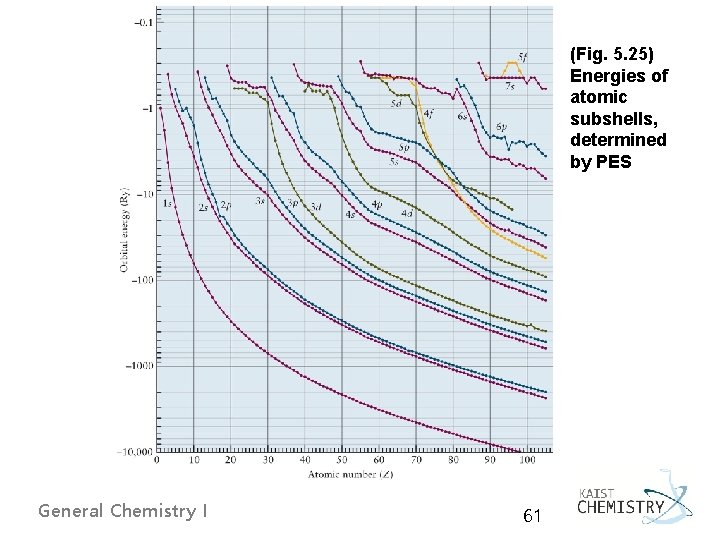
(Fig. 5. 25) Energies of atomic subshells, determined by PES General Chemistry I 61

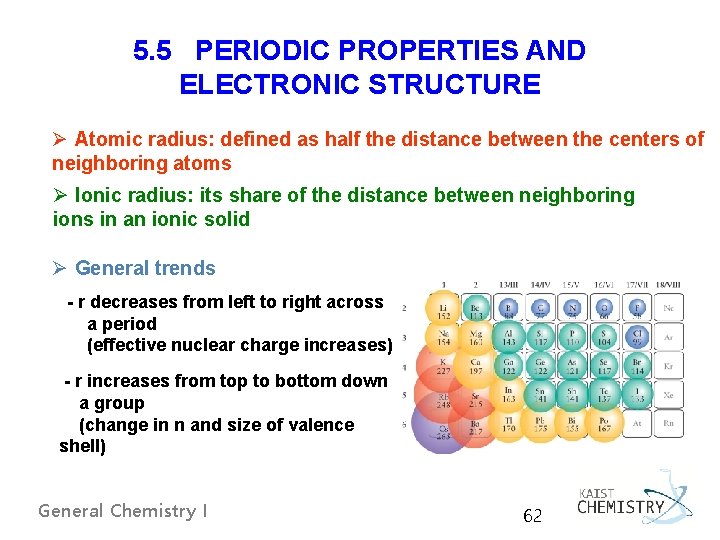
5. 5 PERIODIC PROPERTIES AND ELECTRONIC STRUCTURE Ø Atomic radius: defined as half the distance between the centers of neighboring atoms Ø Ionic radius: its share of the distance between neighboring ions in an ionic solid Ø General trends - r decreases from left to right across a period (effective nuclear charge increases) - r increases from top to bottom down a group (change in n and size of valence shell) General Chemistry I 62

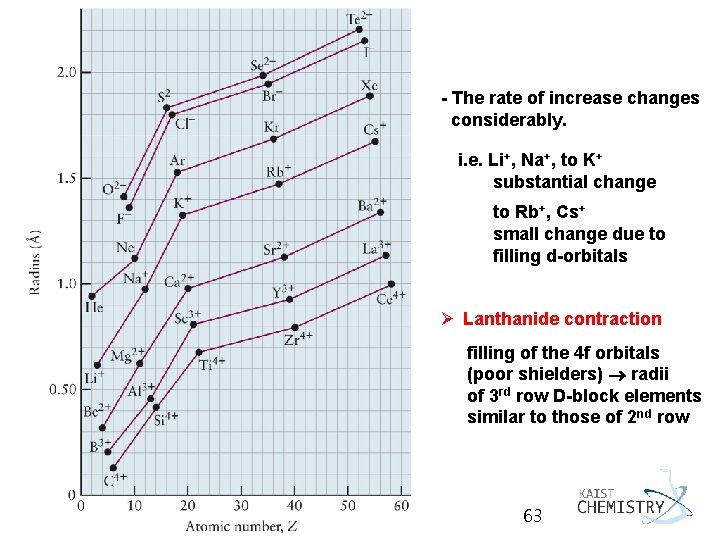
- The rate of increase changes considerably. i. e. Li+, Na+, to K+ substantial change to Rb+, Cs+ small change due to filling d-orbitals Ø Lanthanide contraction filling of the 4 f orbitals (poor shielders) radii of 3 rd row D-block elements similar to those of 2 nd row General Chemistry I 63

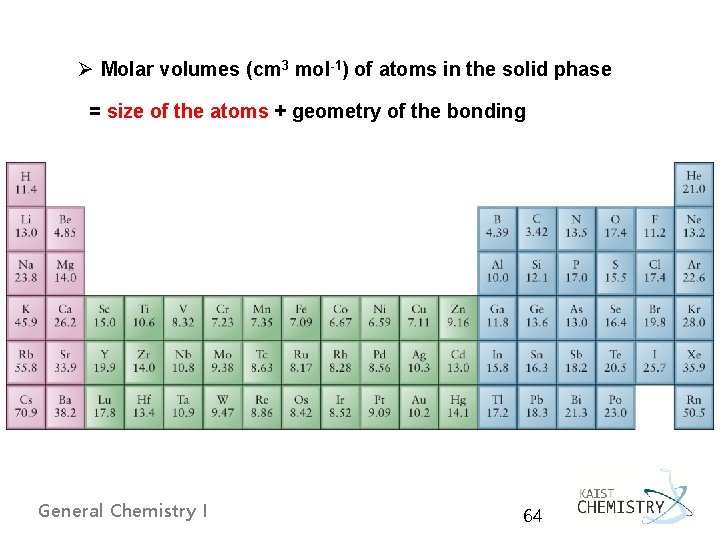
Ø Molar volumes (cm 3 mol-1) of atoms in the solid phase = size of the atoms + geometry of the bonding General Chemistry I 64

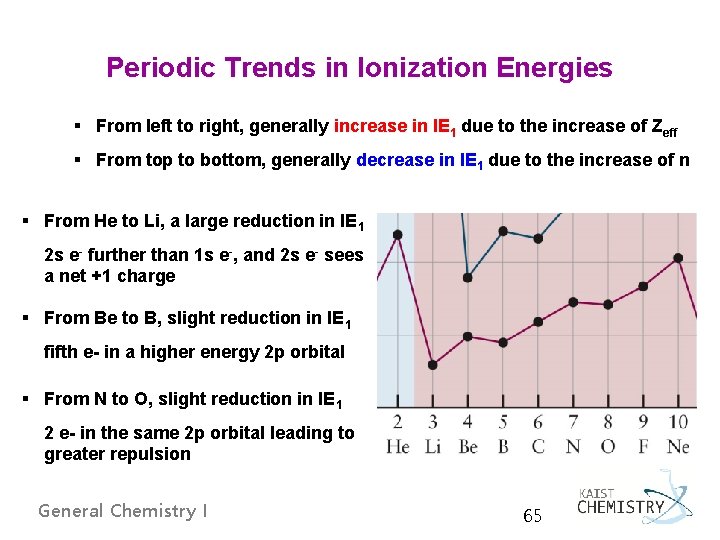
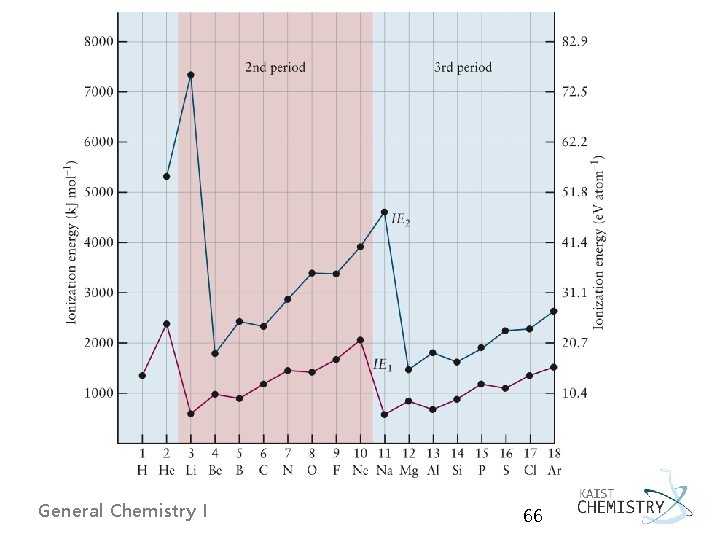
Periodic Trends in Ionization Energies § From left to right, generally increase in IE 1 due to the increase of Zeff § From top to bottom, generally decrease in IE 1 due to the increase of n § From He to Li, a large reduction in IE 1 2 s e- further than 1 s e-, and 2 s e- sees a net +1 charge § From Be to B, slight reduction in IE 1 fifth e- in a higher energy 2 p orbital § From N to O, slight reduction in IE 1 2 e- in the same 2 p orbital leading to greater repulsion General Chemistry I 65

General Chemistry I 66

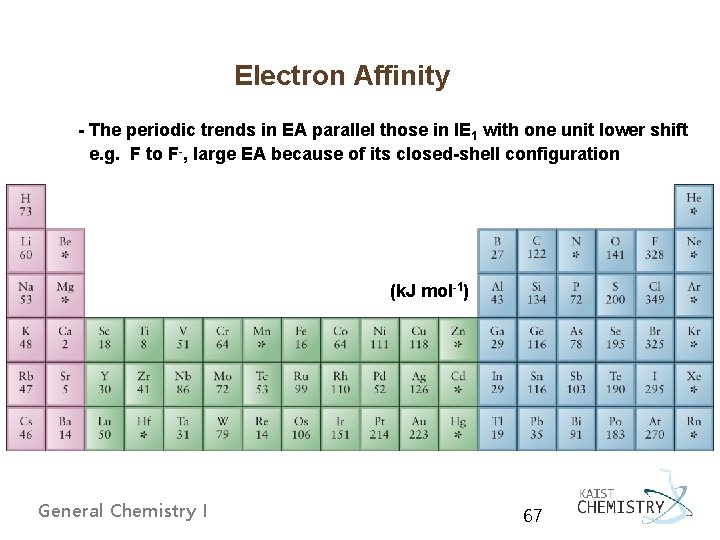
Electron Affinity - The periodic trends in EA parallel those in IE 1 with one unit lower shift e. g. F to F-, large EA because of its closed-shell configuration (k. J mol-1) General Chemistry I 67

10 Problem Sets For Chapter 5, 6, 18, 22, 28, 32, 38, 44, 46, 48, 56 General Chemistry I 68