5 Ionic Solids Structure and Properties a Formula



- Slides: 15

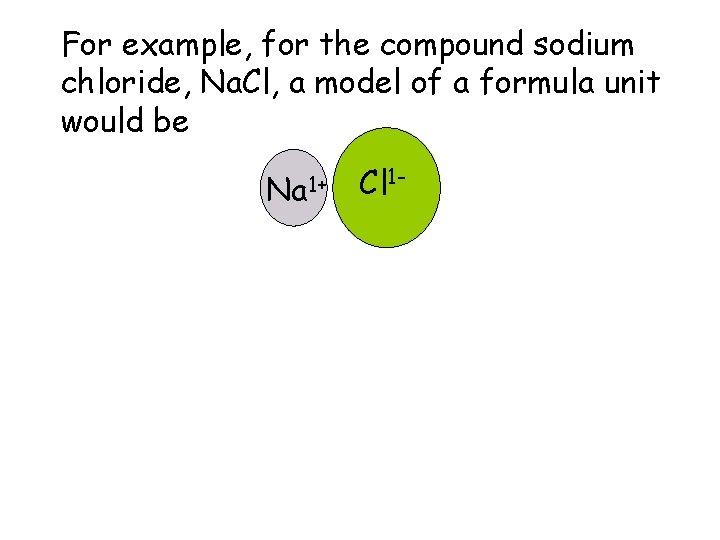
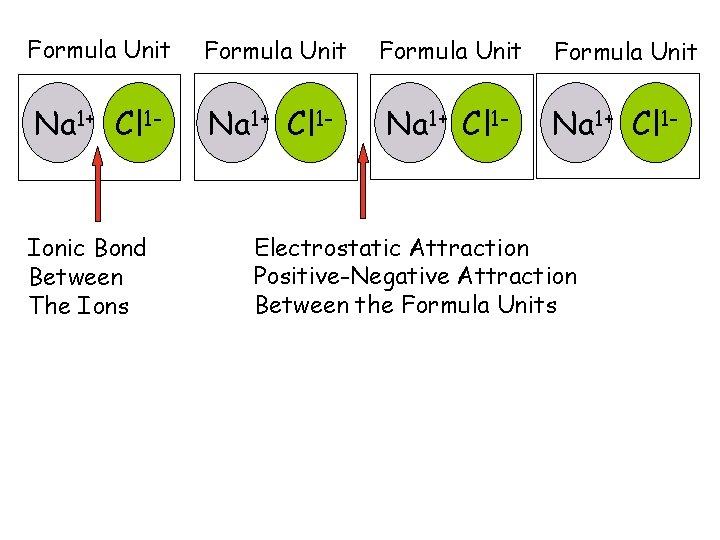
5. Ionic Solids, Structure and Properties a. Formula Units The smallest part of an ionic compound that has all the properties of that compound is called a formula unit.

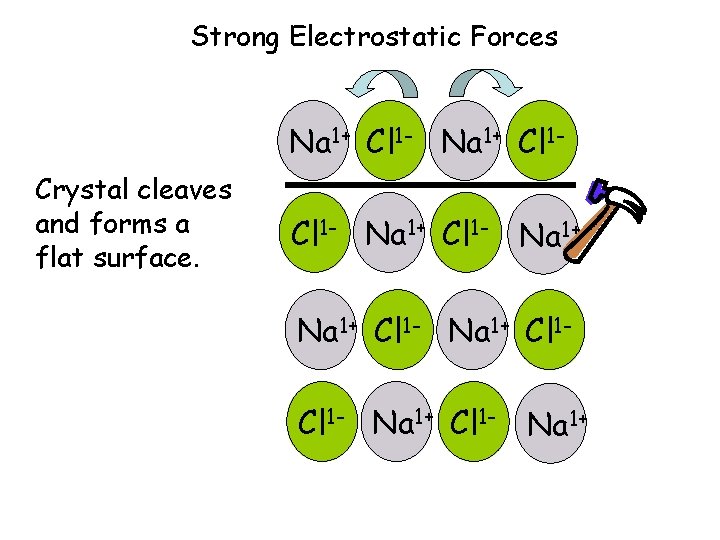
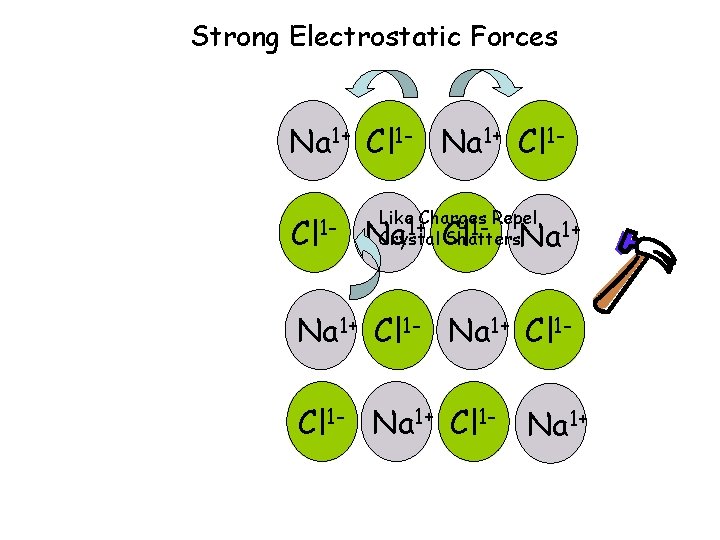
Ionic Compounds • Have high melting points due to large electrostatic attraction, and are not volatile. • Are brittle will shatter or cleave when struck • Conduct electricity when dissolved or molten (liquid) • Binary (2 elements) compounds have “-ide” as a subscript when naming. – IONS move to conduct electricity

For example, for the compound sodium chloride, Na. Cl, a model of a formula unit would be Na 1+ Cl 1 -

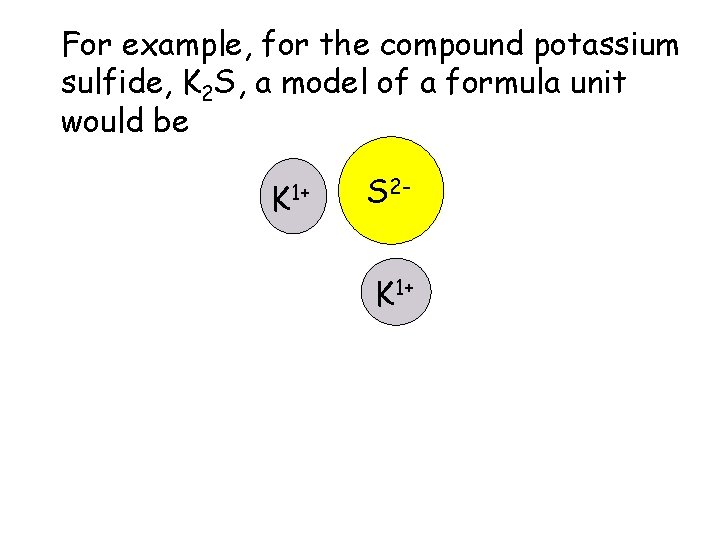
For example, for the compound potassium sulfide, K 2 S, a model of a formula unit would be K 1+ S 2 K 1+

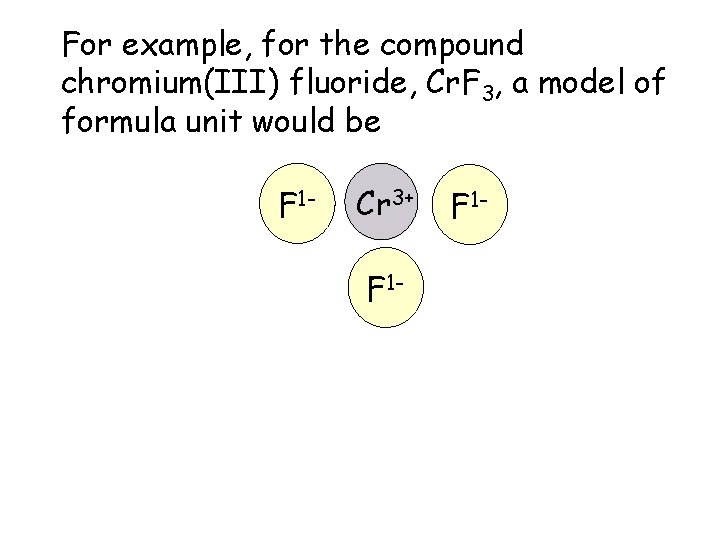
For example, for the compound chromium(III) fluoride, Cr. F 3, a model of formula unit would be F 1 - Cr 3+ F 1 -

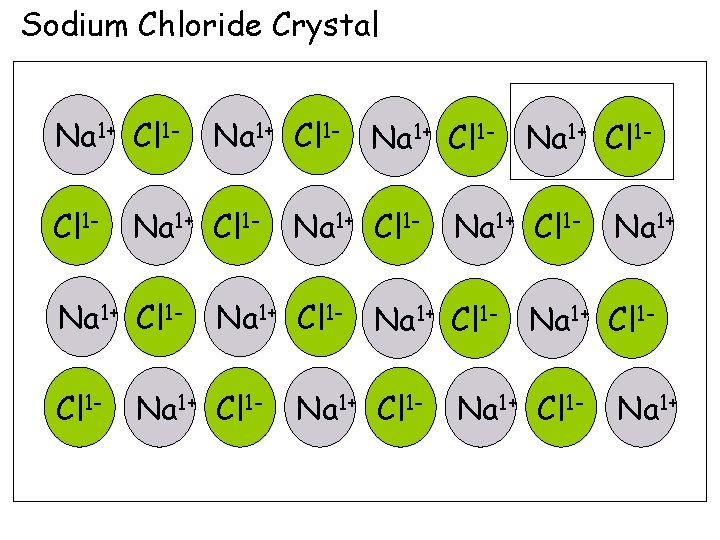
5. Ionic Solids, Structure and Properties b. Crystals An ionic crystal is a large collection of formula units in a an alternating array of positive and negative ions. The positions that the ions occupy and that determine the observable shape of the crystal are called the lattice points.

Formula Unit Na 1+ Cl 1 - Ionic Bond Between The Ions Formula Unit Na 1+ Cl 1 - Electrostatic Attraction Positive-Negative Attraction Between the Formula Units

Sodium Chloride Crystal Na 1+ Cl 1 Cl 1 - Na 1+ Cl 1 - Na 1+ Cl 1 - Na 1+ Cl 1 - Na 1+

5. Ionic Solids c. Properties - generally hard - but can be cleaved or broken - low volatility - high melting and boiling points - do not conduct electricity in the solid state - does conduct electricity when melted or when placed in water

Strong Electrostatic Forces Na 1+ Cl 1 - Na 1+ Cl 1 Crystal cleaves and forms a flat surface. Cl 1 - Na 1+ Cl 1 - Na 1+

Strong Electrostatic Forces Na 1+ Cl 1 - Like Charges Repel 1+ 1 Crystal Shatters Na Cl Na 1+ Cl 1 - Na 1+

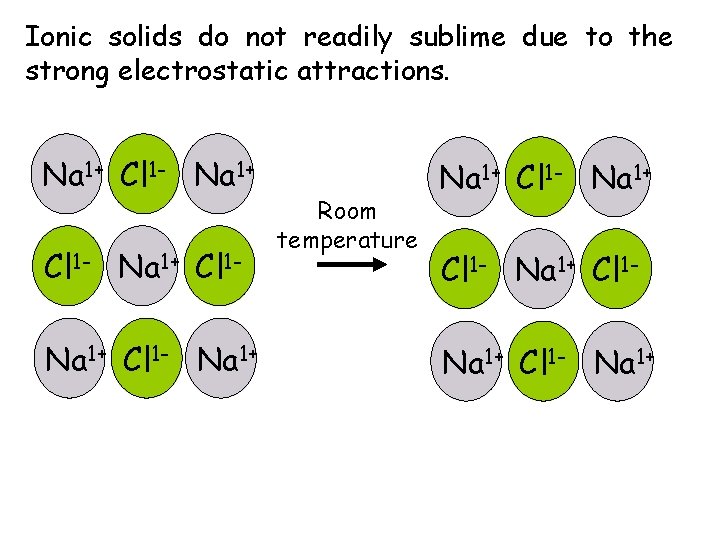
Ionic solids do not readily sublime due to the strong electrostatic attractions. Na 1+ Cl 1 - Na 1+ Room temperature Na 1+ Cl 1 - Na 1+

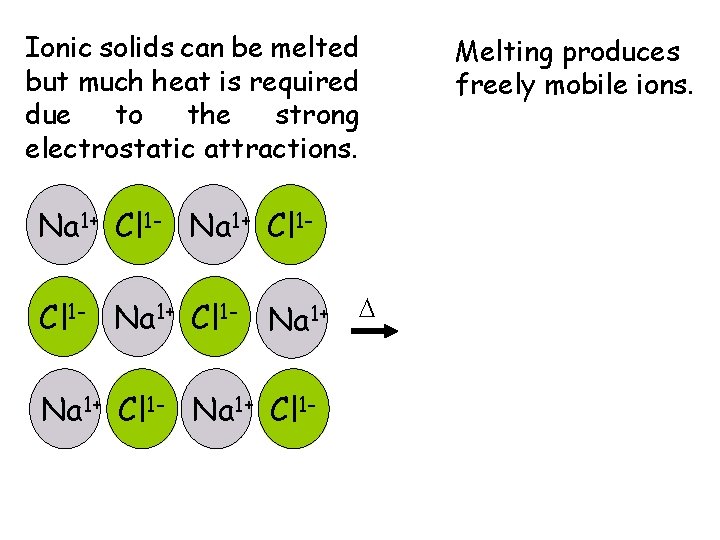
Ionic solids can be melted but much heat is required due to the strong electrostatic attractions. Na 1+ Cl 1 - Na 1+ D Na 1+ Cl 1 - Melting produces freely mobile ions.

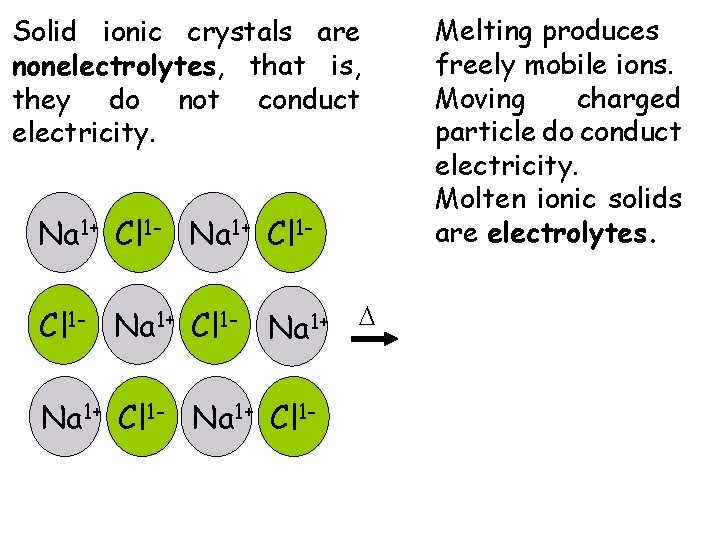
Solid ionic crystals are nonelectrolytes, that is, they do not conduct electricity. Na 1+ Cl 1 - Na 1+ D Na 1+ Cl 1 - Melting produces freely mobile ions. Moving charged particle do conduct electricity. Molten ionic solids are electrolytes.

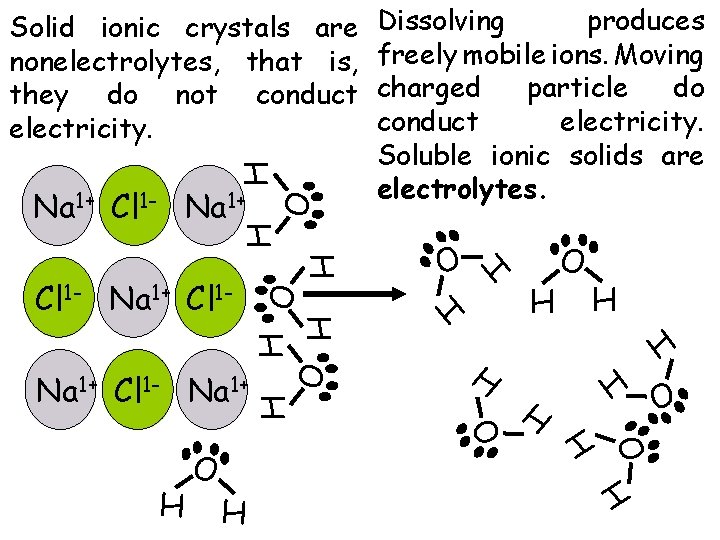
H O H O H Na 1+ Cl 1 - Na 1+ H O H Cl 1 - Na 1+ Cl 1 - O H H H O H H Na 1+ Cl 1 - Na 1+ Dissolving produces freely mobile ions. Moving charged particle do conduct electricity. Soluble ionic solids are electrolytes. H Solid ionic crystals are nonelectrolytes, that is, they do not conduct electricity.