5 Formulas and Names of Ionic Compounds Ionic
- Slides: 9

5. Formulas and Names of Ionic Compounds

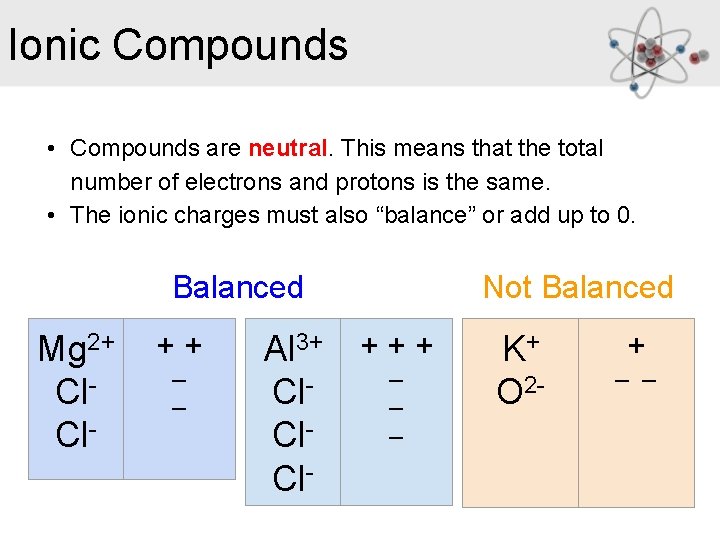
Ionic Compounds • Compounds are neutral. This means that the total number of electrons and protons is the same. • The ionic charges must also “balance” or add up to 0. Balanced Mg 2+ Cl. Cl- ++ _ _ Al 3+ Cl. Cl- Not Balanced +++ _ _ _ K+ O 2 - + _ _

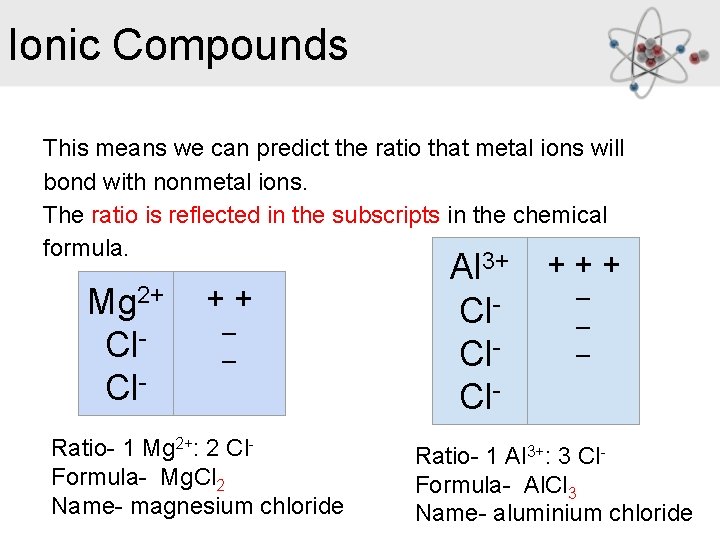
Ionic Compounds This means we can predict the ratio that metal ions will bond with nonmetal ions. The ratio is reflected in the subscripts in the chemical formula. Al 3+ + +_ + Mg 2+ +_+ Cl_ _ Cl. Cl- Cl Ratio- 1 Mg 2+: 2 Cl. Formula- Mg. Cl 2 Name- magnesium chloride Ratio- 1 Al 3+: 3 Cl. Formula- Al. Cl 3 Name- aluminium chloride

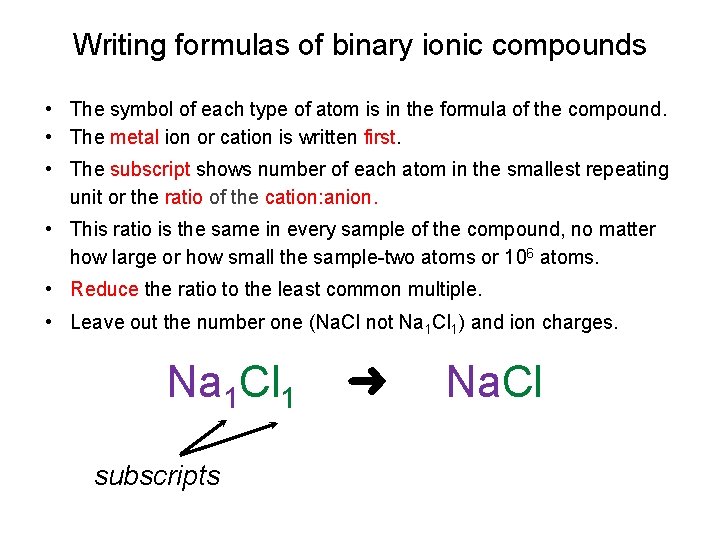
Writing formulas of binary ionic compounds • The symbol of each type of atom is in the formula of the compound. • The metal ion or cation is written first. • The subscript shows number of each atom in the smallest repeating unit or the ratio of the cation: anion. • This ratio is the same in every sample of the compound, no matter how large or how small the sample-two atoms or 106 atoms. • Reduce the ratio to the least common multiple. • Leave out the number one (Na. Cl not Na 1 Cl 1) and ion charges. Na 1 Cl 1 subscripts ➜ Na. Cl

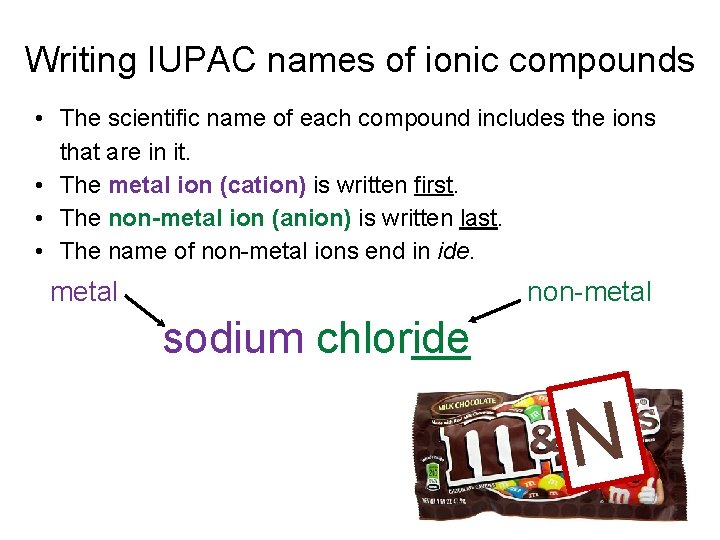
Writing IUPAC names of ionic compounds • The scientific name of each compound includes the ions that are in it. • The metal ion (cation) is written first. • The non-metal ion (anion) is written last. • The name of non-metal ions end in ide. non-metal sodium chloride N

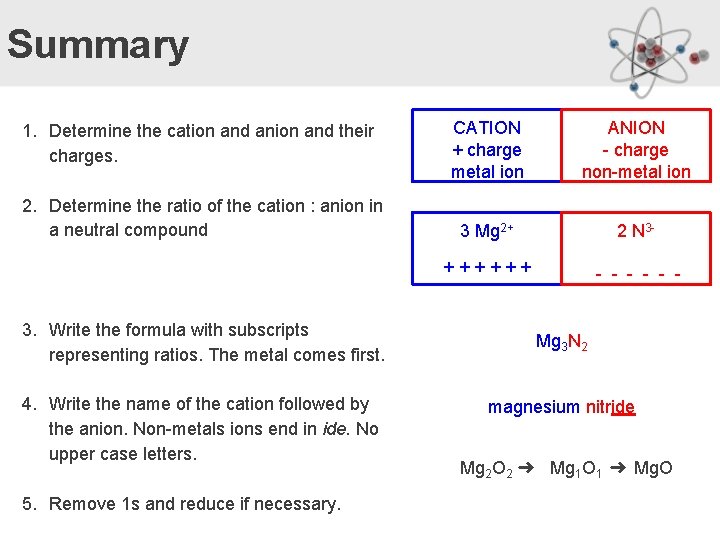
Summary 1. Determine the cation and anion and their charges. 2. Determine the ratio of the cation : anion in a neutral compound 3. Write the formula with subscripts representing ratios. The metal comes first. 4. Write the name of the cation followed by the anion. Non-metals ions end in ide. No upper case letters. 5. Remove 1 s and reduce if necessary. CATION + charge metal ion ANION - charge non-metal ion 3 Mg 2+ 2 N 3 - ++++++ - - - Mg 3 N 2 magnesium nitride Mg 2 O 2 ➜ Mg 1 O 1 ➜ Mg. O

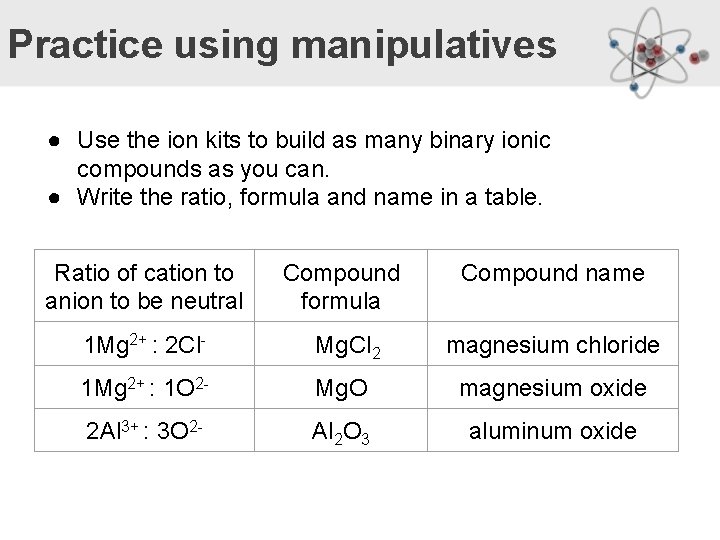
Practice using manipulatives ● Use the ion kits to build as many binary ionic compounds as you can. ● Write the ratio, formula and name in a table. Ratio of cation to anion to be neutral Compound formula Compound name 1 Mg 2+ : 2 Cl- Mg. Cl 2 magnesium chloride 1 Mg 2+ : 1 O 2 - Mg. O magnesium oxide 2 Al 3+ : 3 O 2 - Al 2 O 3 aluminum oxide

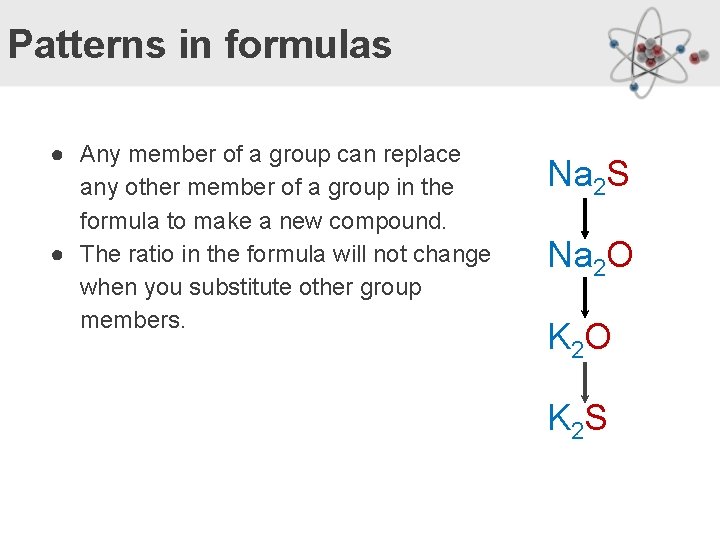
Patterns in formulas ● Any member of a group can replace any other member of a group in the formula to make a new compound. ● The ratio in the formula will not change when you substitute other group members. Na 2 S Na 2 O K 2 S

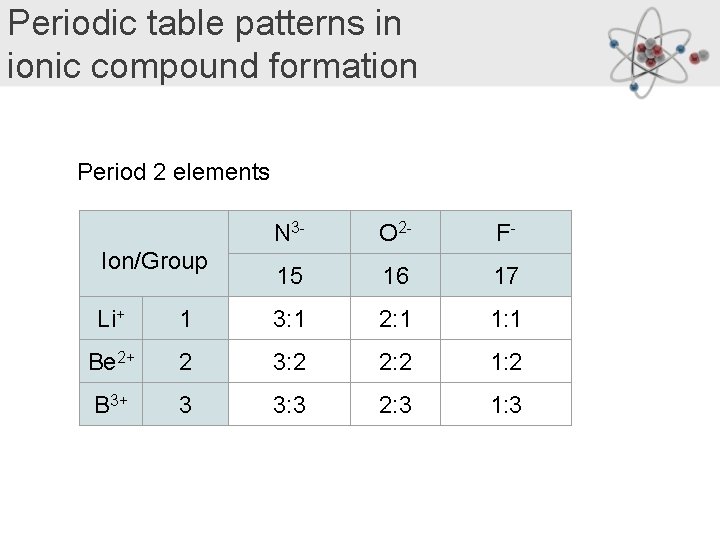
Periodic table patterns in ionic compound formation Period 2 elements Ion/Group N 3 - O 2 - F- 15 16 17 Li+ 1 3: 1 2: 1 1: 1 Be 2+ 2 3: 2 2: 2 1: 2 B 3+ 3 3: 3 2: 3 1: 3