5 Common Reaction Types synthesis decomposition combustion single


5 Common Reaction Types: -synthesis -decomposition -combustion -single displacement -double displacement synthesis - means to make something or build Example: synthetic material, photosynthesis A + B = AB decomposition- means to something down §starts out as 1 whole and breaks into 2 or more §EX: decomposing (dead) animals or garbage AB = A + B
5 Common Reaction Types: -synthesis -decomposition -combustion -single displacement -double displacement Combustion-burning §always has CO 2 as a product A(fuel) + O 2 = H 2 O + CO 2 §actual example: C 3 H 8 + 5 O 2 = 4 H 2 O + 3 CO 2

5 Common Reaction Types: -synthesis -decomposition -combustion -single displacement -double displacement single displacment- 1 “switch” §one element switches or hops to another partner AB + C = AC + B §Cl switches to pair w/ Ca in the product 2 Na. Cl + Ca = Ca. Cl 2 + 2 Na §Like a divorce and a remarriage
5 Common Reaction Types: -synthesis -decomposition -combustion -single displacement -double displacement double displacment- 2 “switch” § 2 elements switch or change partners AB + XY = AY + BX §Na & So 4 both switches partners in the product Na 2 SO 4 + Be. O= Be. SO 4 + Na 2 O
5 Types of Chemical Reactions Guess which common reaction type: 4 Fe + 3 O 2 Fe 2 O 3 2 substances 1 product üsynthesis
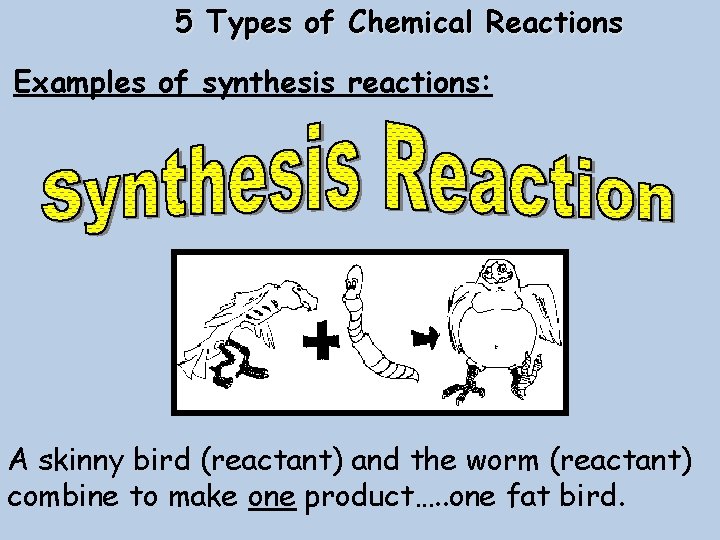
5 Types of Chemical Reactions Examples of synthesis reactions: A skinny bird (reactant) and the worm (reactant) combine to make one product…. . one fat bird.
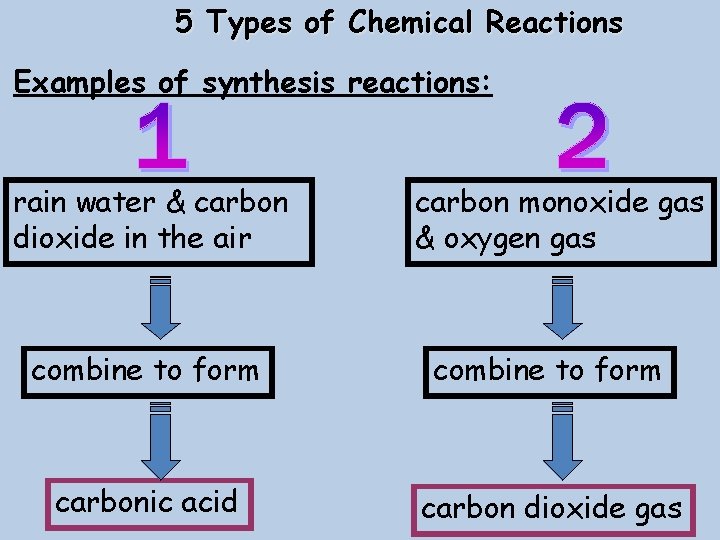
5 Types of Chemical Reactions Examples of synthesis reactions: rain water & carbon dioxide in the air carbon monoxide gas & oxygen gas combine to form carbonic acid carbon dioxide gas
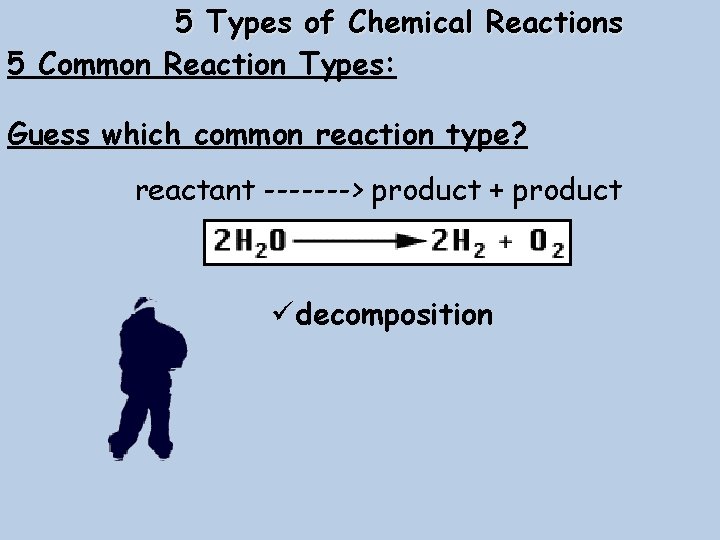
5 Types of Chemical Reactions 5 Common Reaction Types: Guess which common reaction type? reactant -------> product + product üdecomposition
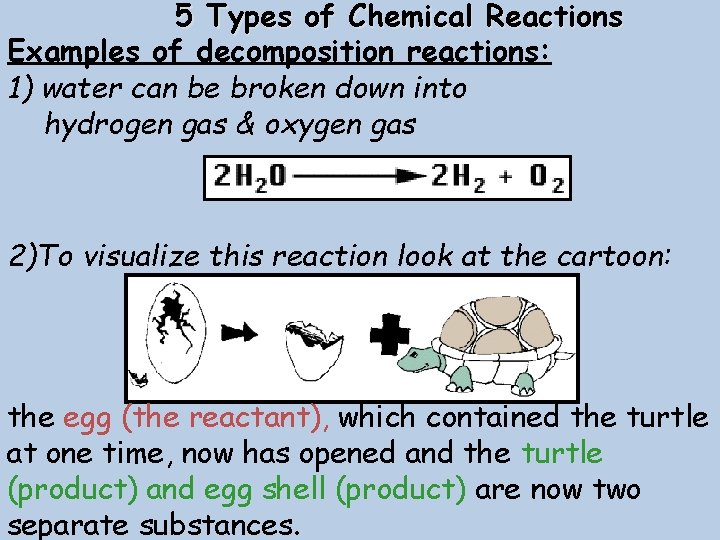
5 Types of Chemical Reactions Examples of decomposition reactions: 1) water can be broken down into hydrogen gas & oxygen gas 2)To visualize this reaction look at the cartoon: the egg (the reactant), which contained the turtle at one time, now has opened and the turtle (product) and egg shell (product) are now two separate substances.
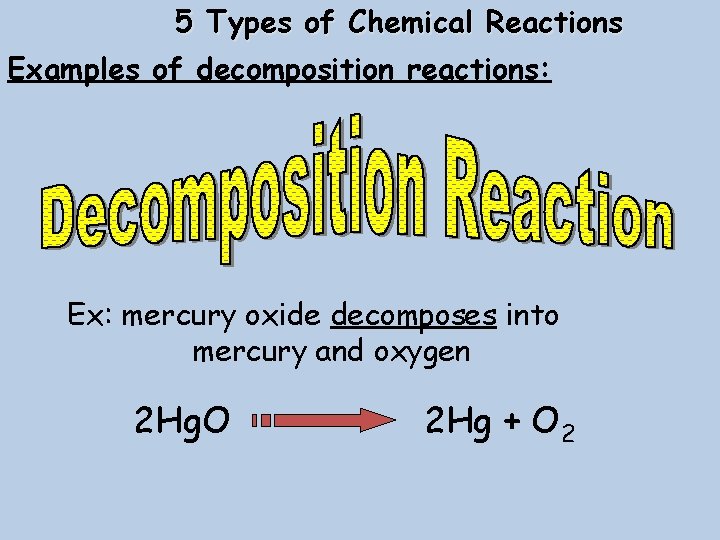
5 Types of Chemical Reactions Examples of decomposition reactions: Ex: mercury oxide decomposes into mercury and oxygen 2 Hg. O 2 Hg + O 2
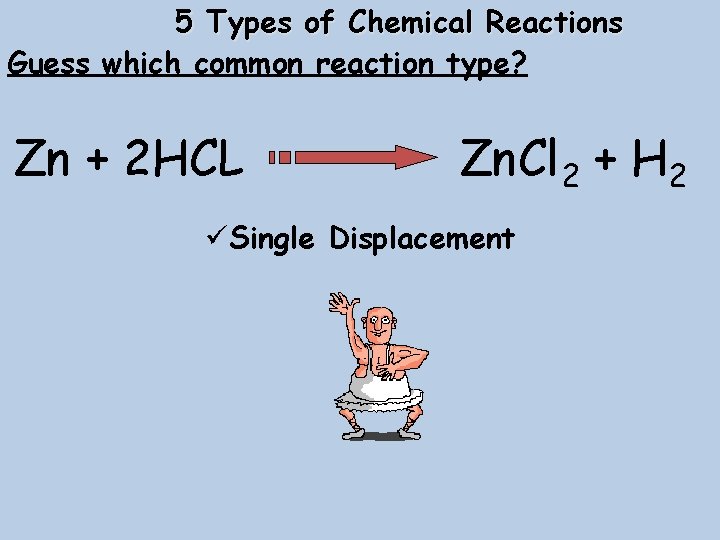
5 Types of Chemical Reactions Guess which common reaction type? Zn + 2 HCL Zn. Cl 2 + H 2 üSingle Displacement
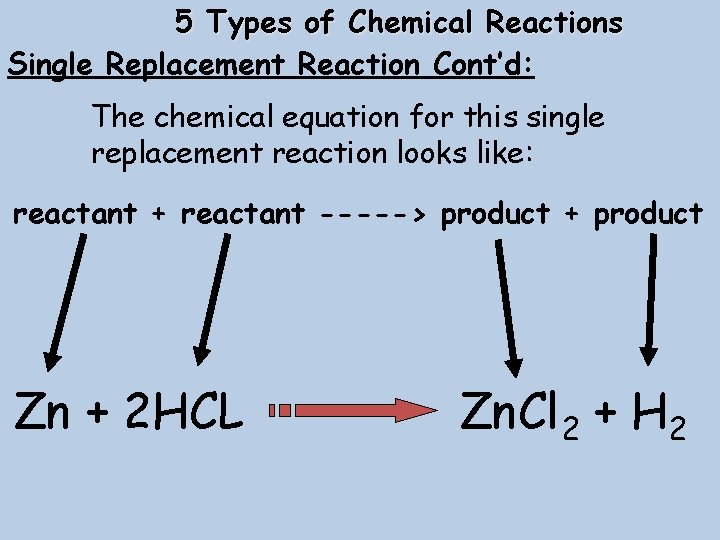
5 Types of Chemical Reactions Single Replacement Reaction Cont’d: The chemical equation for this single replacement reaction looks like: reactant + reactant -----> product + product Zn + 2 HCL Zn. Cl 2 + H 2
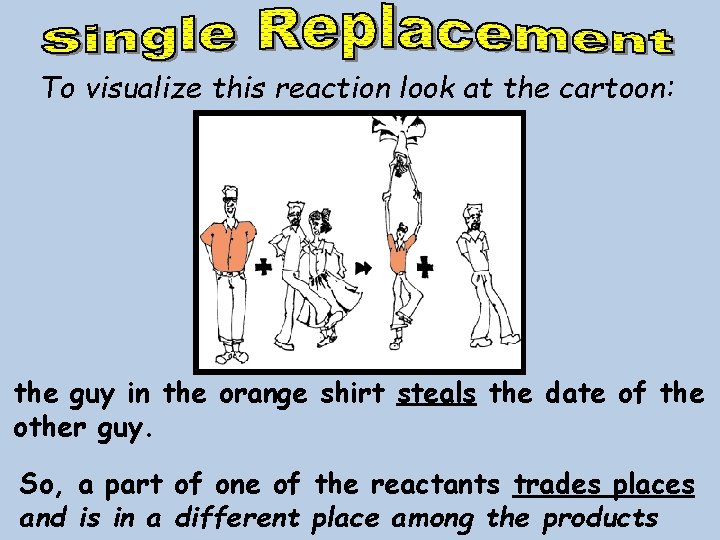
To visualize this reaction look at the cartoon: the guy in the orange shirt steals the date of the other guy. So, a part of one of the reactants trades places and is in a different place among the products
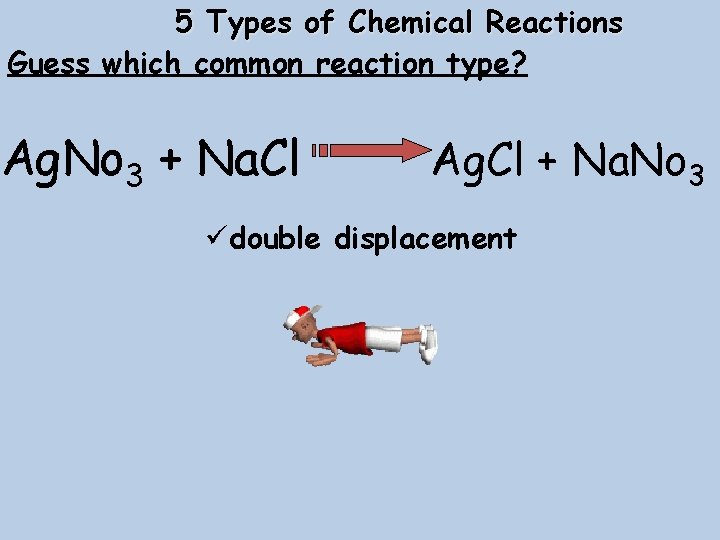
5 Types of Chemical Reactions Guess which common reaction type? Ag. No 3 + Na. Cl Ag. Cl + Na. No 3 üdouble displacement
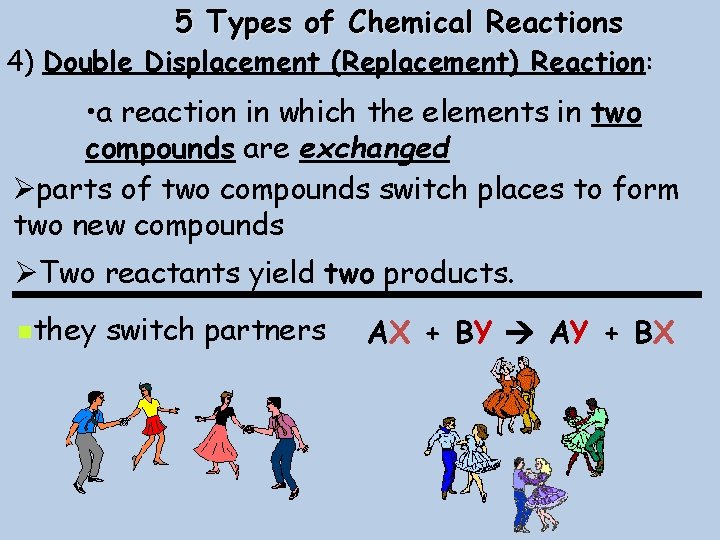
5 Types of Chemical Reactions 4) Double Displacement (Replacement) Reaction: • a reaction in which the elements in two compounds are exchanged Øparts of two compounds switch places to form two new compounds ØTwo reactants yield two products. nthey switch partners AX + BY AY + BX
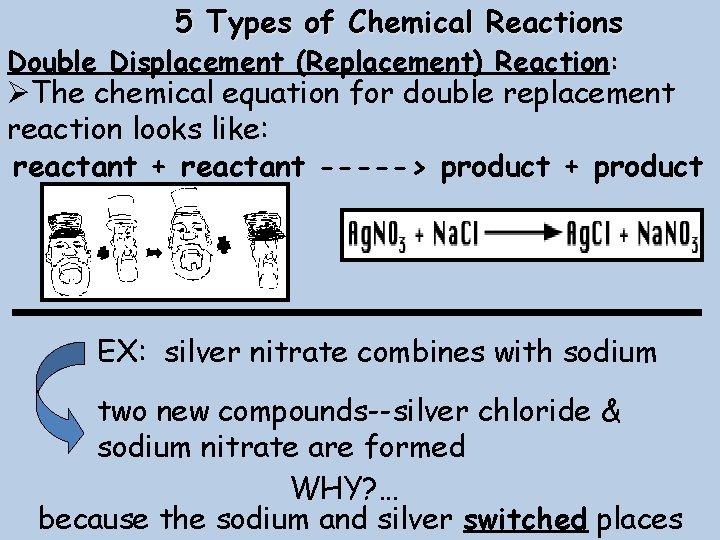
5 Types of Chemical Reactions Double Displacement (Replacement) Reaction: ØThe chemical equation for double replacement reaction looks like: reactant + reactant -----> product + product EX: silver nitrate combines with sodium two new compounds--silver chloride & sodium nitrate are formed WHY? … because the sodium and silver switched places
5 Types of Chemical Reactions 5 Common Reaction Types: Guess which common reaction type? C 7 H 6 O + O 2 ---> CO 2 + H 2 O ücombustion
- Slides: 18