5 9 5 10 Mean Free Path Diffusion




- Slides: 6

5. 9 -5. 10 Mean Free Path, Diffusion and Effusion of Gases, and Real Gases

Mean Free Path • The average distance that a molecule travels between collisions is its MFP. Why could it take a couple of minutes to smell someone’s perfume/cologne from across the room? Even though the large molecules travel in straight-line motion and they travel extremely fast, they collide billions of times with other molecules in the air prior to making a connection with your nasal cavity.

Diffusion and Effusion • Diffusion – the process by which gas molecules spread out in response to a concentration gradient. Which gas diffuses faster, helium or nitrogen, and why? Helium diffuses faster because it is lighter than nitrogen. Gases that are less massive, diffuse faster. • Effusion- the process by which a gas escapes a from a container into a vacuum through a small hole. Would a balloon filled with hydrogen or helium remain inflated longer? How, about with air? Why? The balloon filled with helium would remain inflated longer that the balloon filled with hydrogen, but the balloon with air would remain inflated the longest. This is due to the fact that gases with a lower molar mass escape more quickly than those with a higher molar mass.

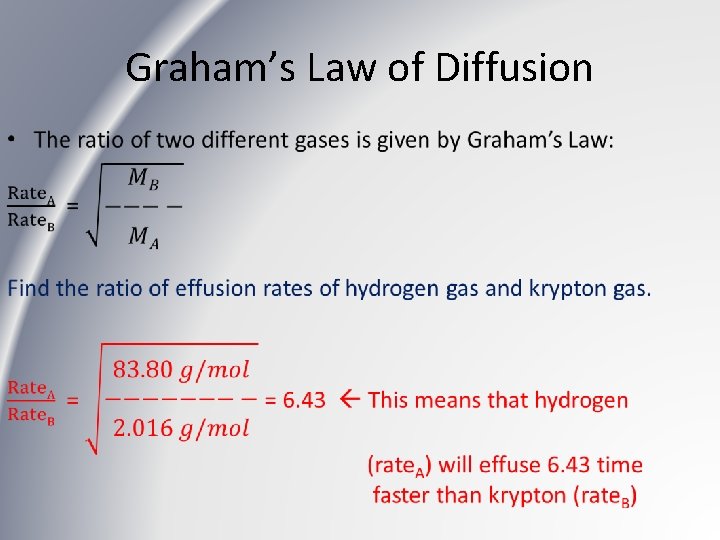
Graham’s Law of Diffusion •

Real Gases • At STP, many real gases exhibit ideal behavior, but it’s also true that real gases do attract to each other. • Under high pressure and/or low temperature, the attraction between gas particles become stronger due to the intermolecular forces between them. Since these attractions are small (weak), they only become attracted if the atoms or molecules are close together. Which gas has a higher boiling point chlorine or fluorine? Why? Chlorine has a higher boiling point because it is a larger molecule. A larger molecule has both more protons as well as more electrons. The migration of electrons around the molecule is the reason that London dispersion forces exist. This migration creates a temporary dipole, and the strength of the force of attraction is proportional to the polarizability of the molecule. As the molecule becomes larger, the amount of electrons increase, polarizability increases, so does the boiling point!
