5 4 Patterns and the Periodic Table Science

5. 4 Patterns and the Periodic Table Science 10
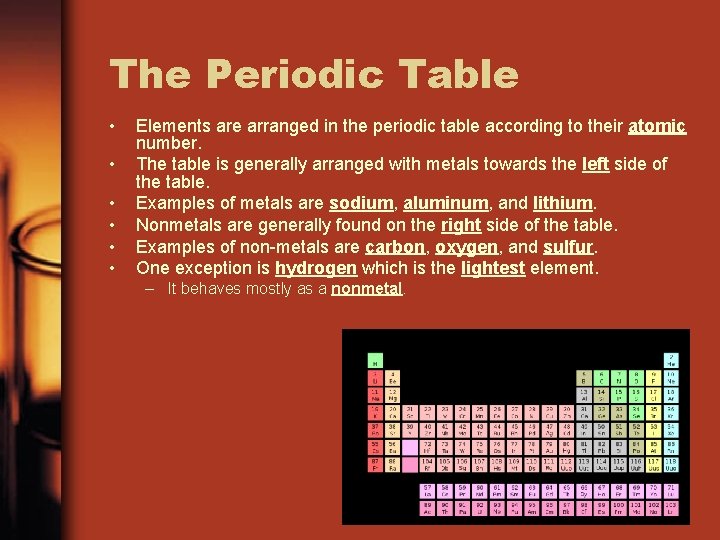
The Periodic Table • • • Elements are arranged in the periodic table according to their atomic number. The table is generally arranged with metals towards the left side of the table. Examples of metals are sodium, aluminum, and lithium. Nonmetals are generally found on the right side of the table. Examples of non-metals are carbon, oxygen, and sulfur. One exception is hydrogen which is the lightest element. – It behaves mostly as a nonmetal.
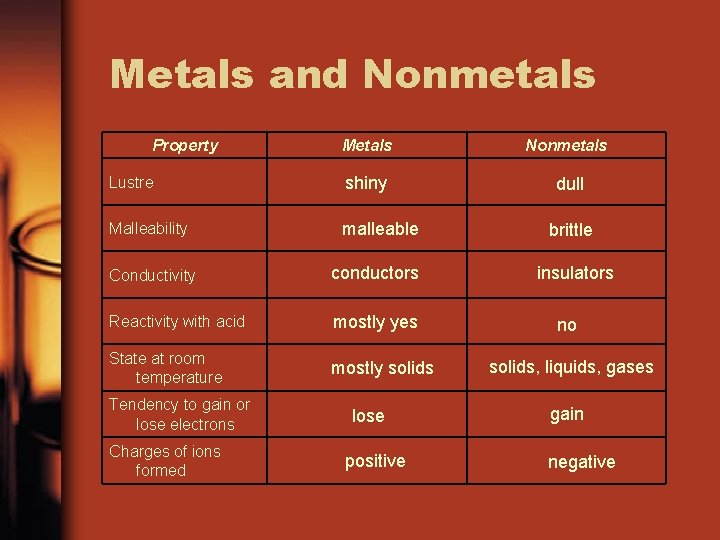
Metals and Nonmetals Property Metals Nonmetals Lustre shiny dull Malleability malleable Conductivity conductors Reactivity with acid mostly yes State at room temperature mostly solids Tendency to gain or lose electrons Charges of ions formed lose positive brittle insulators no solids, liquids, gases gain negative
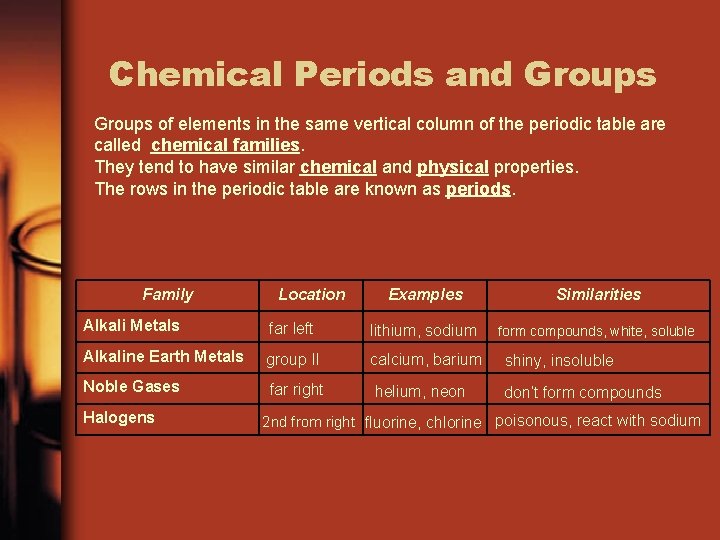
Chemical Periods and Groups of elements in the same vertical column of the periodic table are called chemical families. They tend to have similar chemical and physical properties. The rows in the periodic table are known as periods. Family Location Examples Similarities form compounds, white, soluble Alkali Metals far left lithium, sodium Alkaline Earth Metals group II calcium, barium shiny, insoluble Noble Gases far right helium, neon don’t form compounds Halogens 2 nd from right fluorine, chlorine poisonous, react with sodium
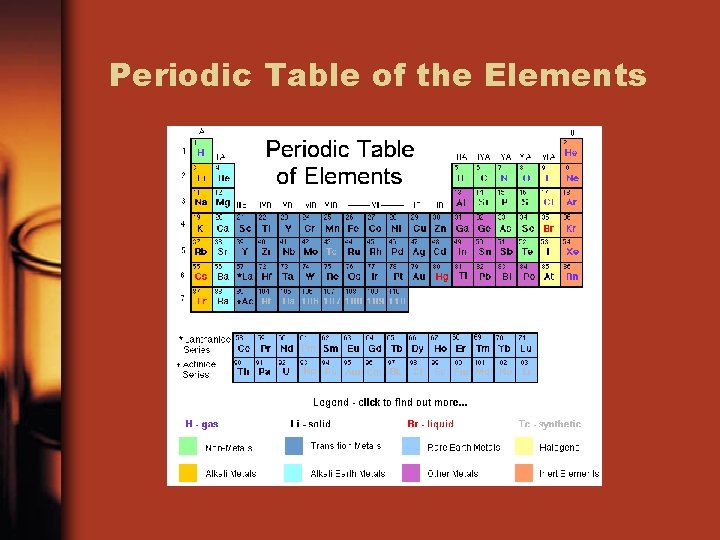
Periodic Table of the Elements
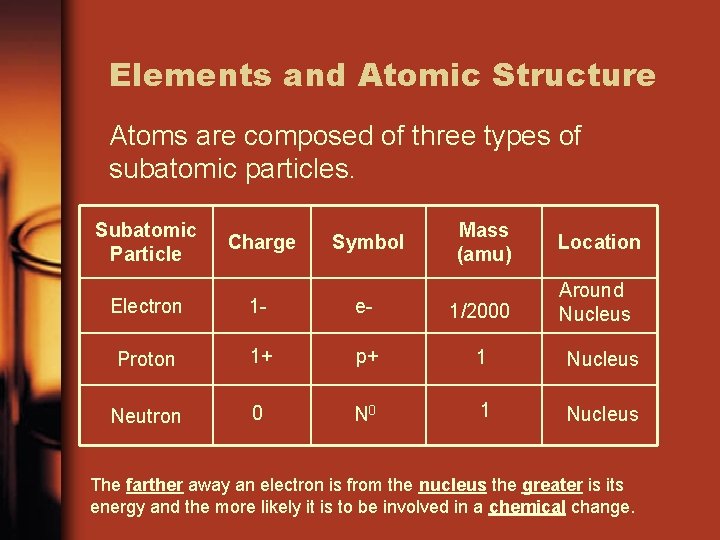
Elements and Atomic Structure Atoms are composed of three types of subatomic particles. Subatomic Particle Charge Symbol Mass (amu) Location Around Nucleus Electron 1 - e- 1/2000 Proton 1+ p+ 1 Nucleus Neutron 0 N 0 1 Nucleus The farther away an electron is from the nucleus the greater is its energy and the more likely it is to be involved in a chemical change.
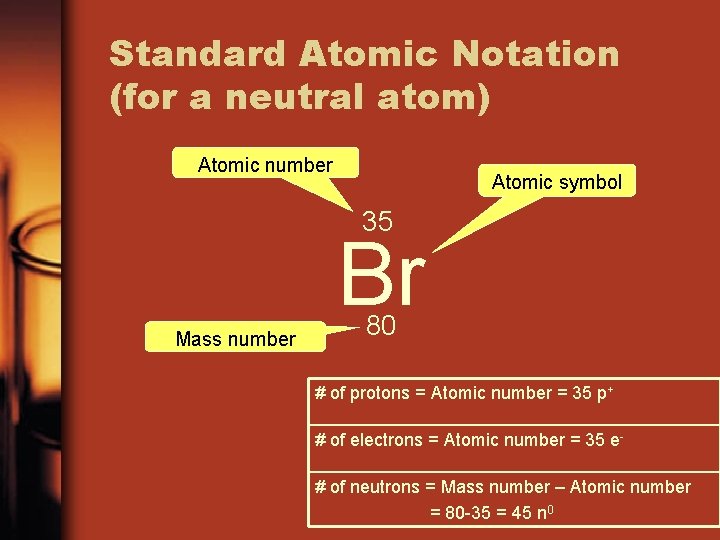
Standard Atomic Notation (for a neutral atom) Atomic number Atomic symbol 35 Br Mass number 80 # of protons = Atomic number = 35 p+ # of electrons = Atomic number = 35 e# of neutrons = Mass number – Atomic number = 80 -35 = 45 n 0

The Bohr-Rutherford Model Nucleus – contains protons and neutrons Electrons – circle the nucleus in orbits – each electron orbit has a definite or maximum amount of electrons. – the first orbit has two electrons, the second orbit holds eight electrons, and the third orbit holds no more than eight electrons. 2 4 He Helium Atom • 2 positive protons in nucleus • 2 neutral neutrons in nucleus • 2 negatively charged electrons in the first energy level
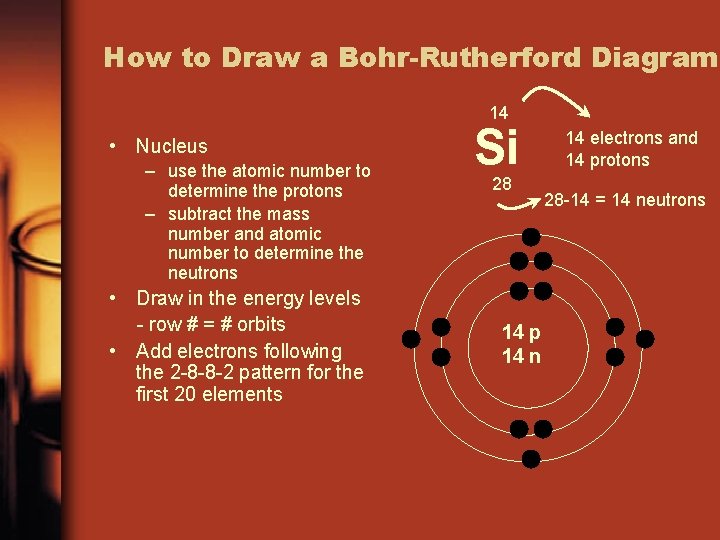
How to Draw a Bohr-Rutherford Diagram 14 • Nucleus – use the atomic number to determine the protons – subtract the mass number and atomic number to determine the neutrons • Draw in the energy levels - row # = # orbits • Add electrons following the 2 -8 -8 -2 pattern for the first 20 elements Si 28 14 p 14 n 14 electrons and 14 protons 28 -14 = 14 neutrons

Homework Pg. 187 #1 -9 Mr. Dee Video Clip: Periodic Table
- Slides: 10