5 4 Electron Configuration redo Electron Configuration distribution
5. 4 – Electron Configuration (redo)
Electron Configuration distribution Electrons Atoms or Ions Ionization Energy
Quantify Coulomb’s Law
Multi-electron ions or atoms Ionization Energy Shells, Subshells and Orbitals
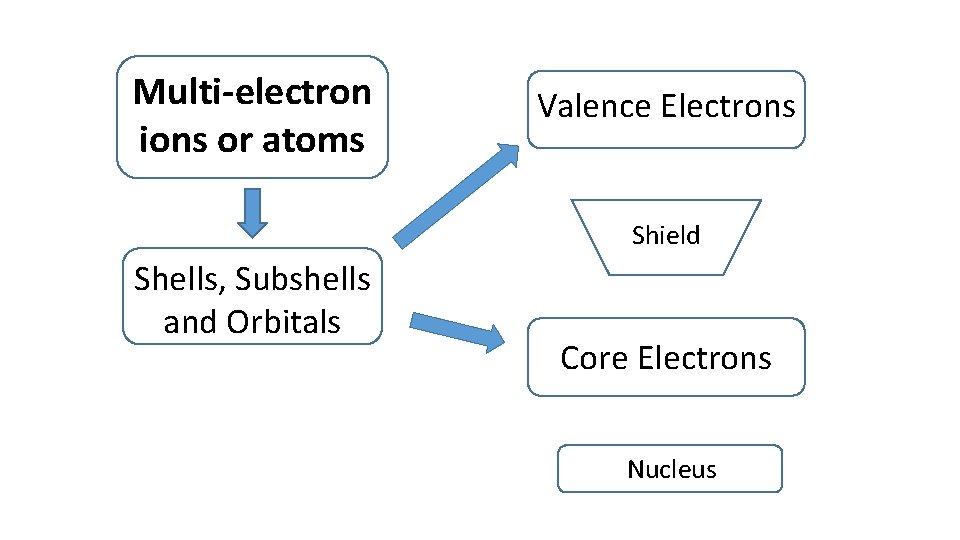
Multi-electron ions or atoms Valence Electrons Shield Shells, Subshells and Orbitals Core Electrons Nucleus
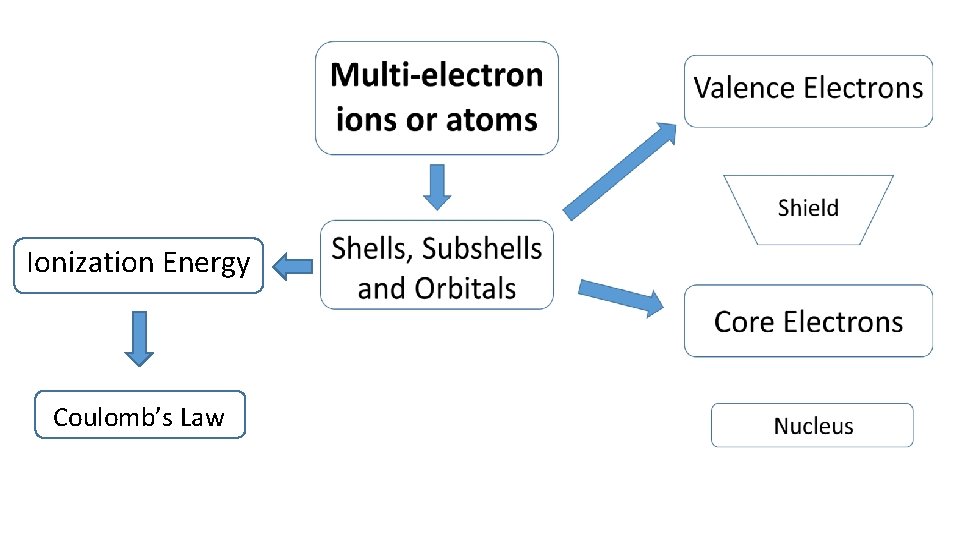
Ionization Energy Coulomb’s Law
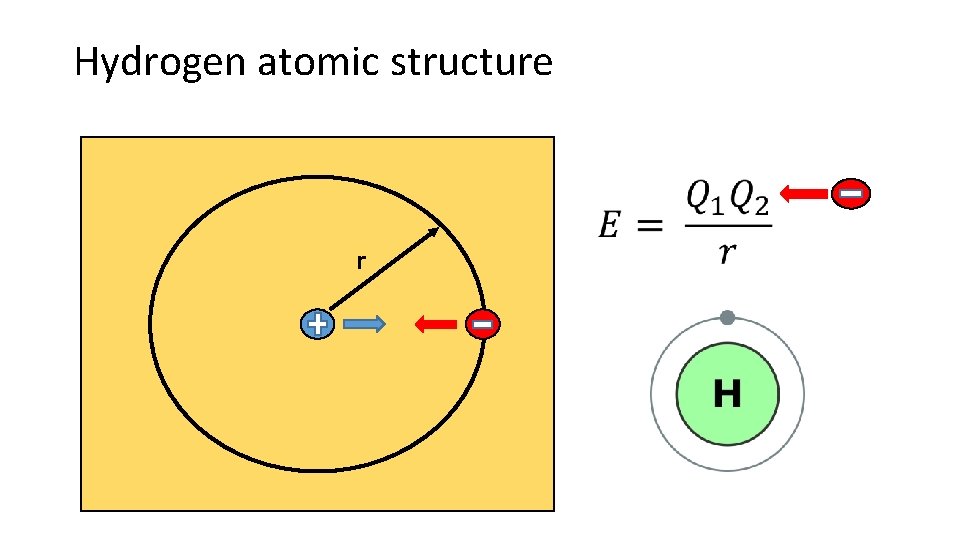
Hydrogen atomic structure r
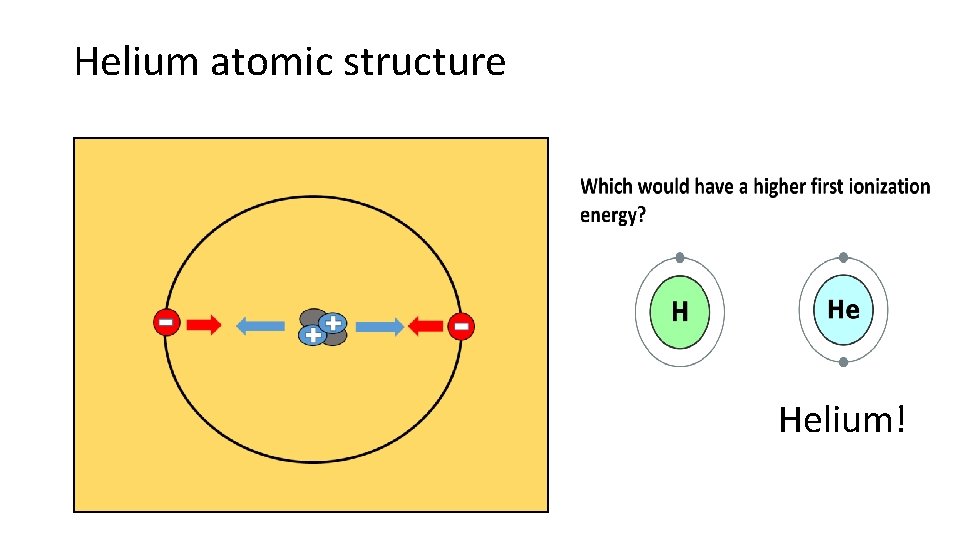
Helium atomic structure Helium!
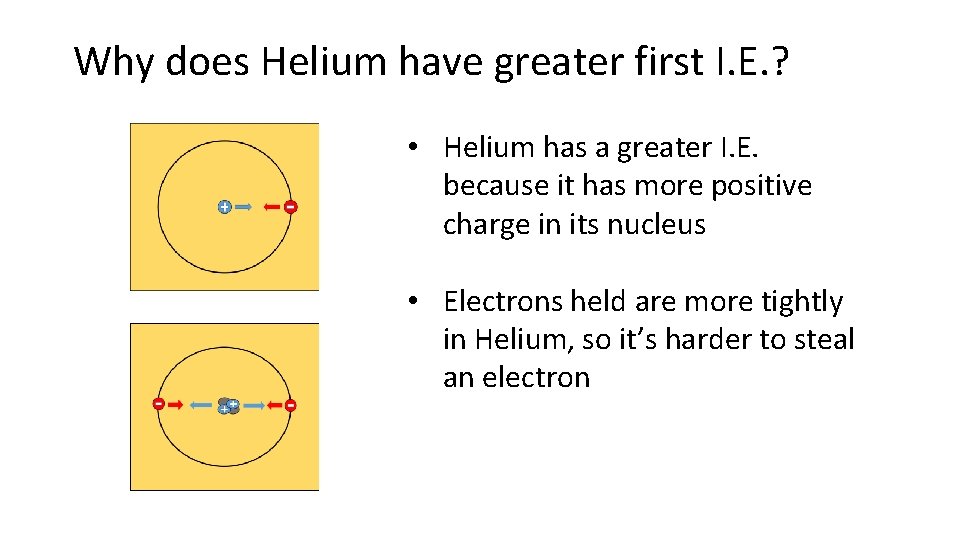
Why does Helium have greater first I. E. ? • Helium has a greater I. E. because it has more positive charge in its nucleus • Electrons held are more tightly in Helium, so it’s harder to steal an electron
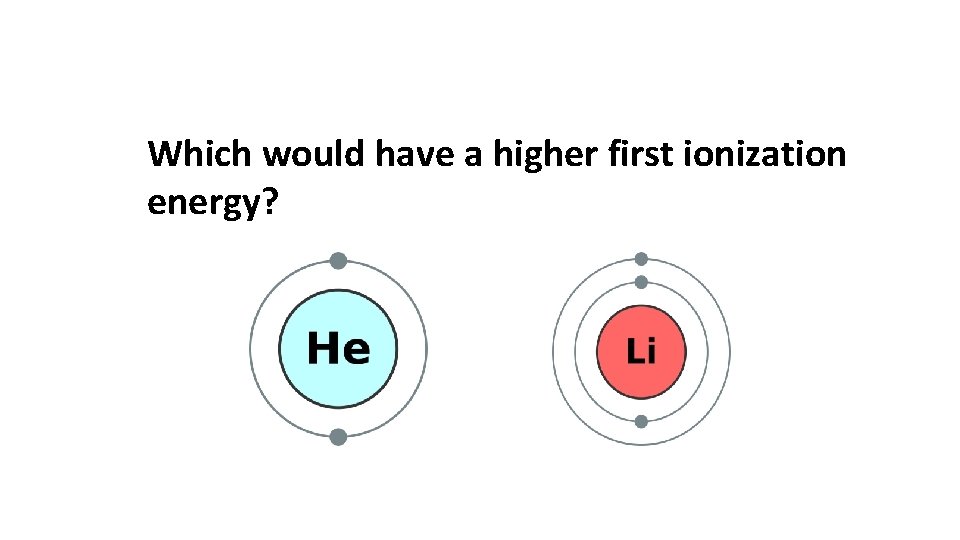
Which would have a higher first ionization energy?
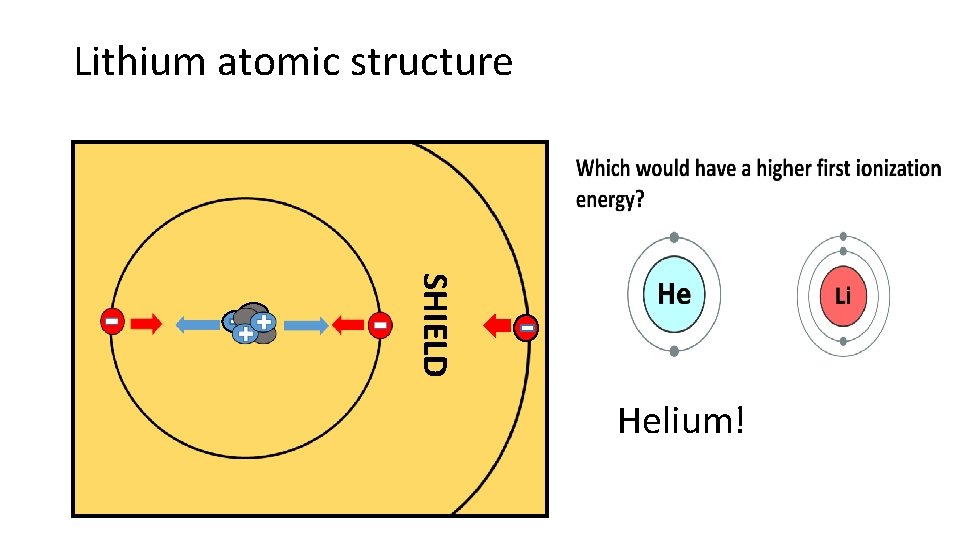
Lithium atomic structure SHIELD Helium!
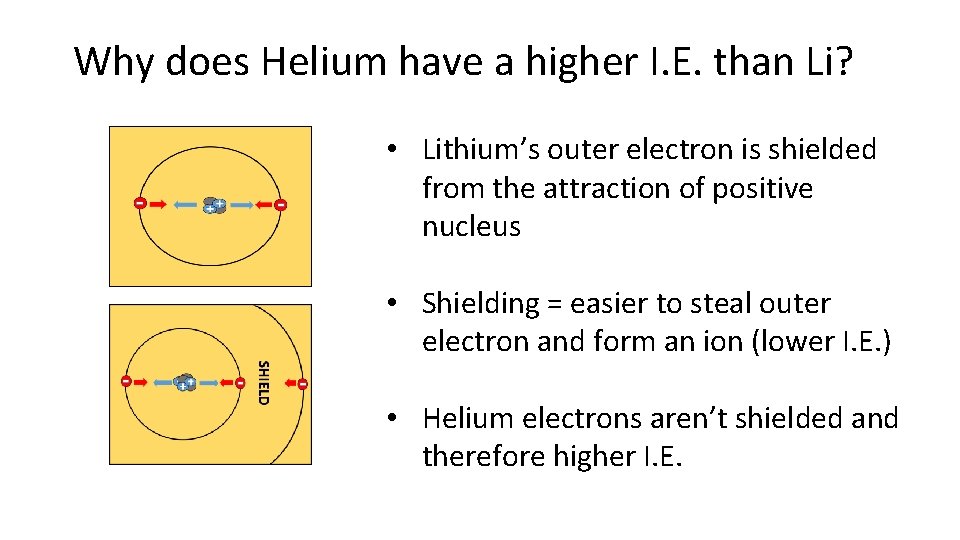
Why does Helium have a higher I. E. than Li? • Lithium’s outer electron is shielded from the attraction of positive nucleus • Shielding = easier to steal outer electron and form an ion (lower I. E. ) • Helium electrons aren’t shielded and therefore higher I. E.

Which would have a higher first ionization energy?
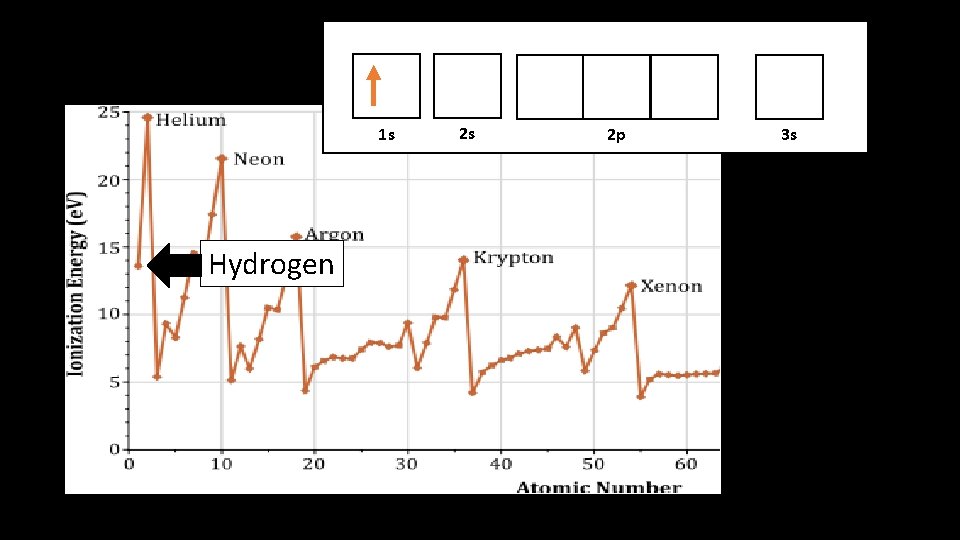
1 s 1 s Hydrogen 2 s 2 p 3 s
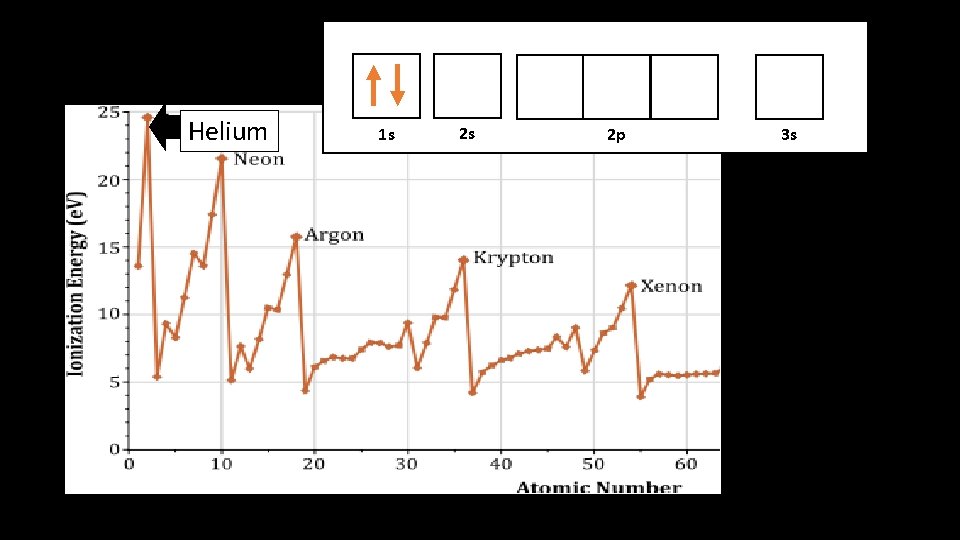
1 s Helium 1 s 2 s 2 p 3 s
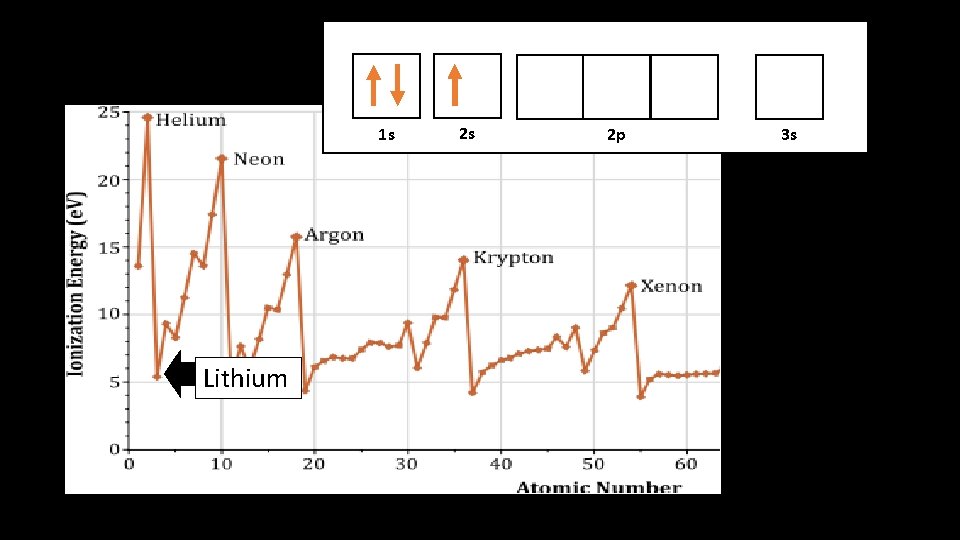
1 s 1 s Lithium 2 s 2 p 3 s
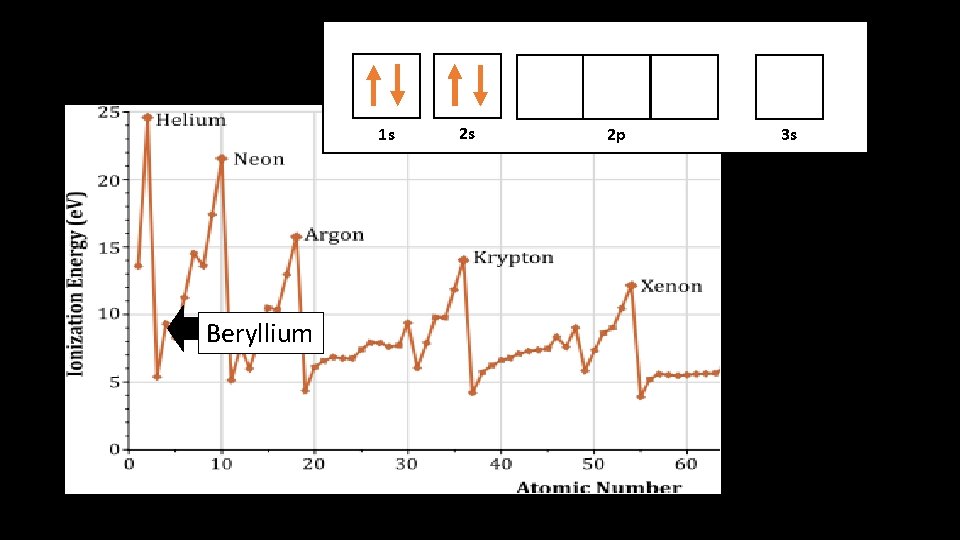
1 s 1 s Beryllium 2 s 2 p 3 s
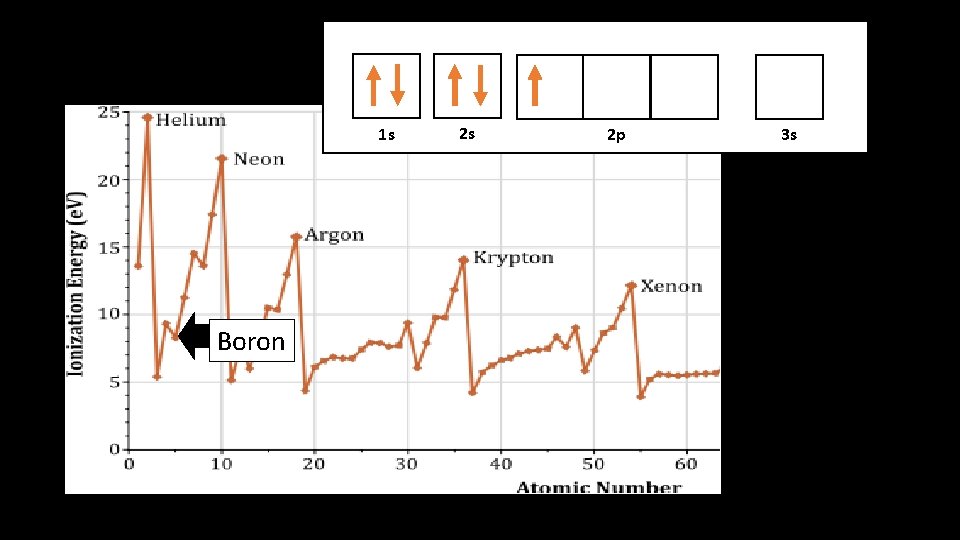
1 s 1 s Boron 2 s 2 p 3 s
1 s 1 s Carbon 2 s 2 p 3 s
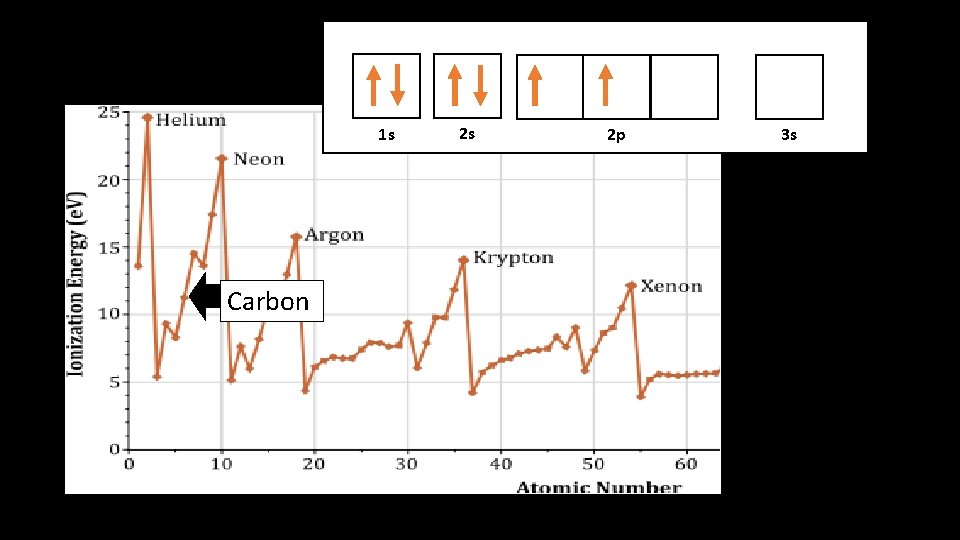
1 s 1 s Carbon 2 s 2 p 3 s
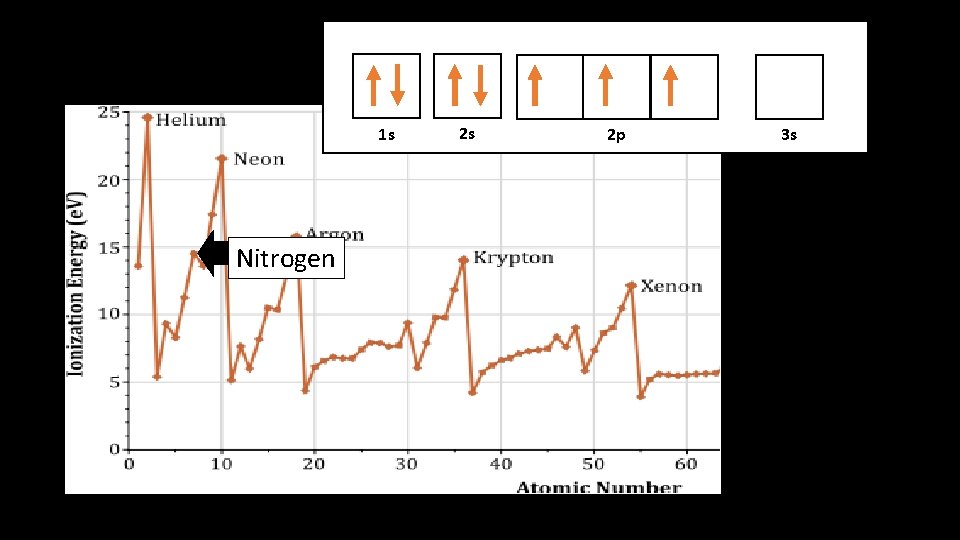
1 s 1 s Nitrogen 2 s 2 p 3 s
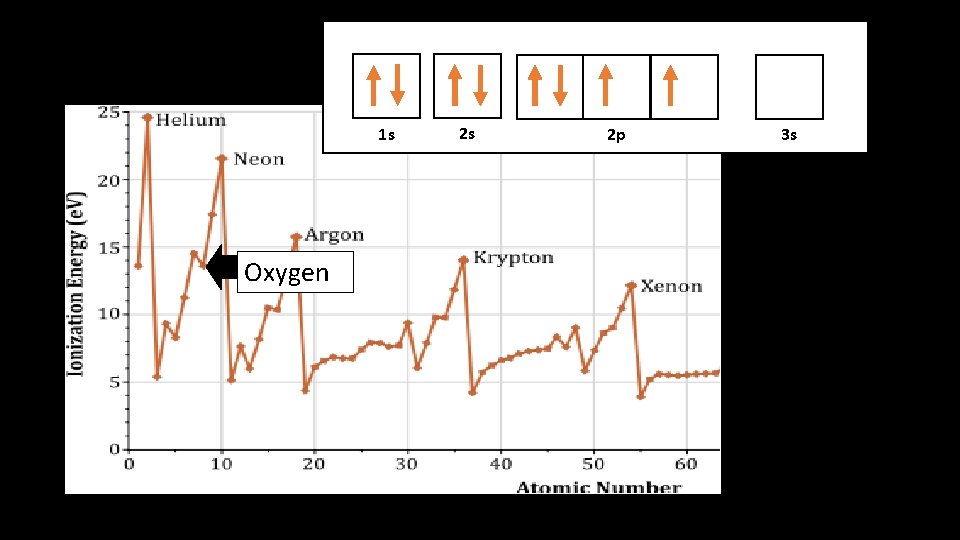
1 s 1 s Oxygen 2 s 2 p 3 s
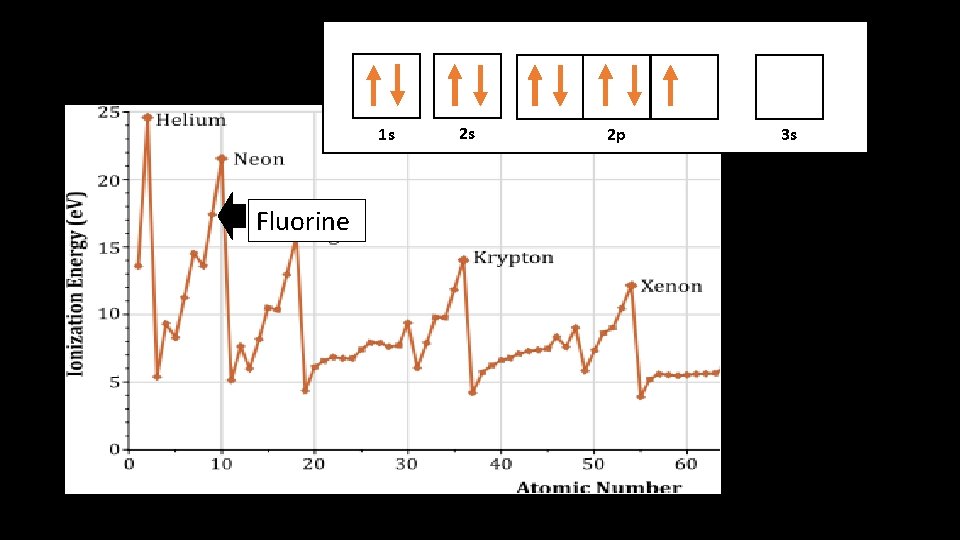
1 s 1 s Fluorine 2 s 2 p 3 s
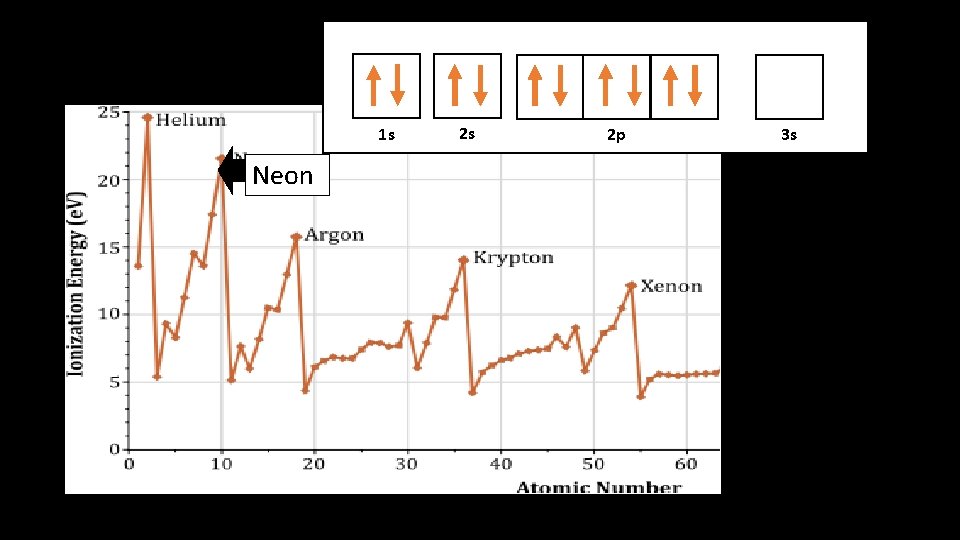
1 s 1 s Neon 2 s 2 p 3 s
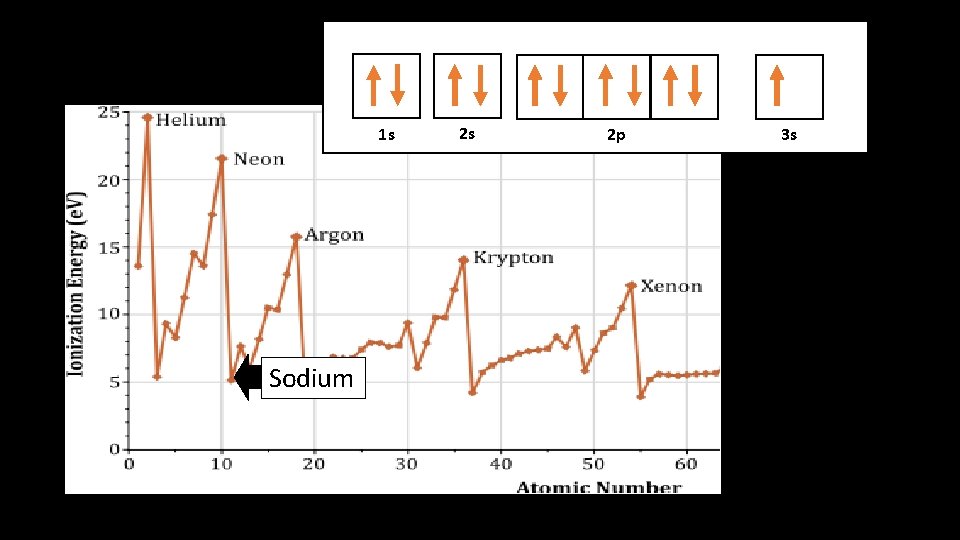
1 s 1 s Sodium 2 s 2 p 3 s
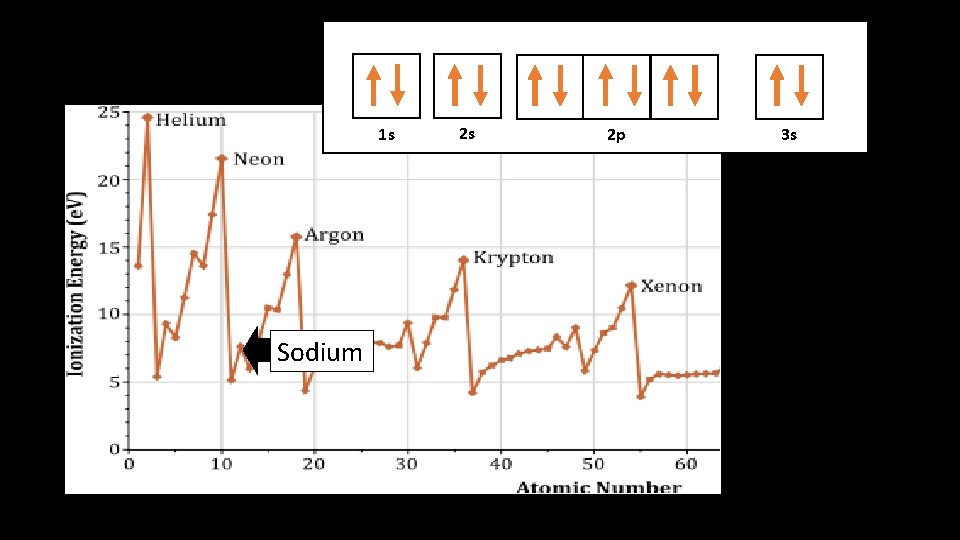
1 s 1 s Sodium 2 s 2 p 3 s
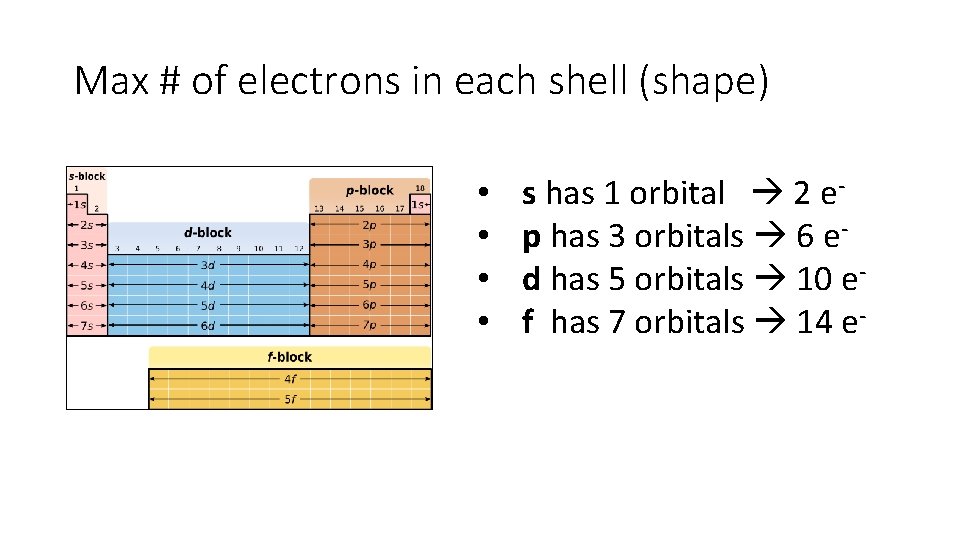
Max # of electrons in each shell (shape) • • s has 1 orbital 2 ep has 3 orbitals 6 ed has 5 orbitals 10 ef has 7 orbitals 14 e-
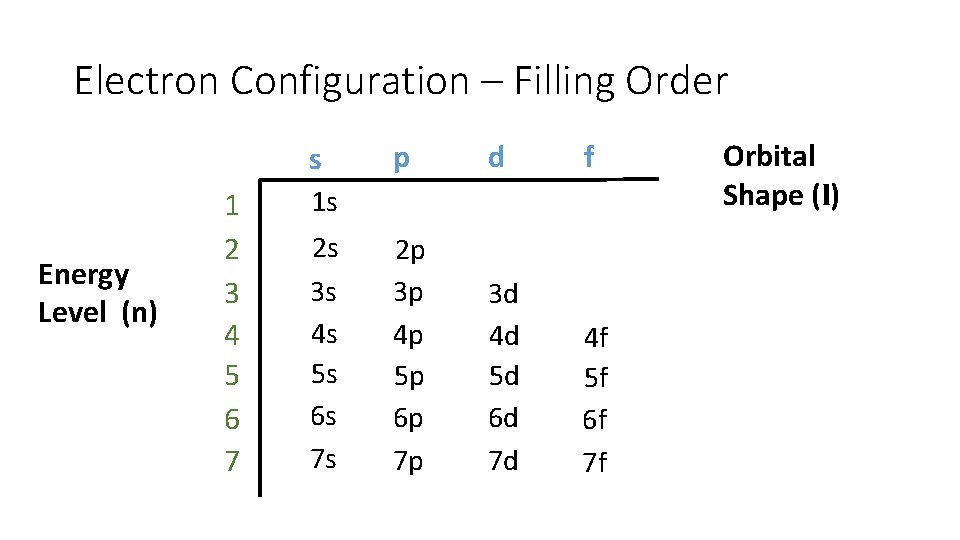
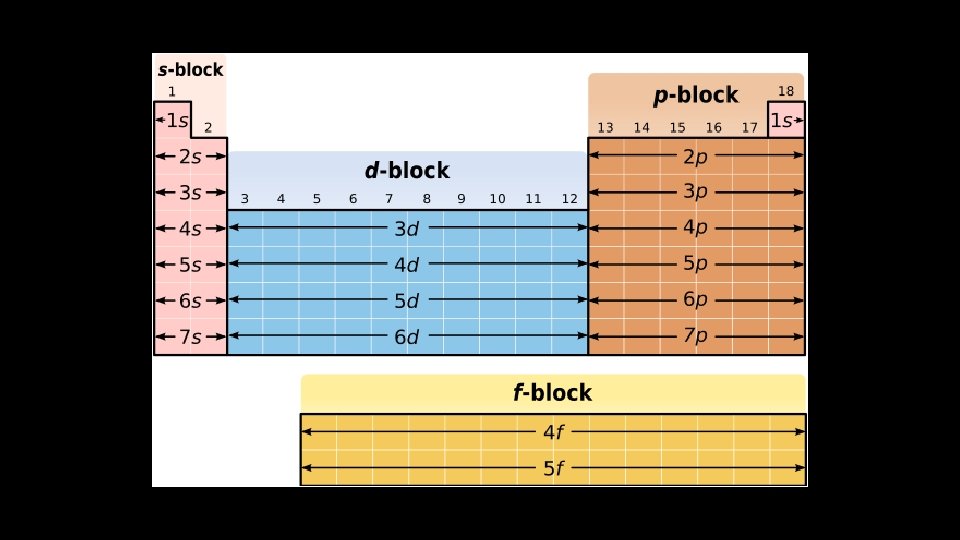
Electron Configuration – Filling Order s Energy Level (n) 1 2 3 4 5 6 7 p d f 2 p 3 p 4 p 5 p 6 p 7 p 3 d 4 d 5 d 6 d 7 d 4 f 5 f 6 f 7 f 1 s 2 s 3 s 4 s 5 s 6 s 7 s Orbital Shape (l)
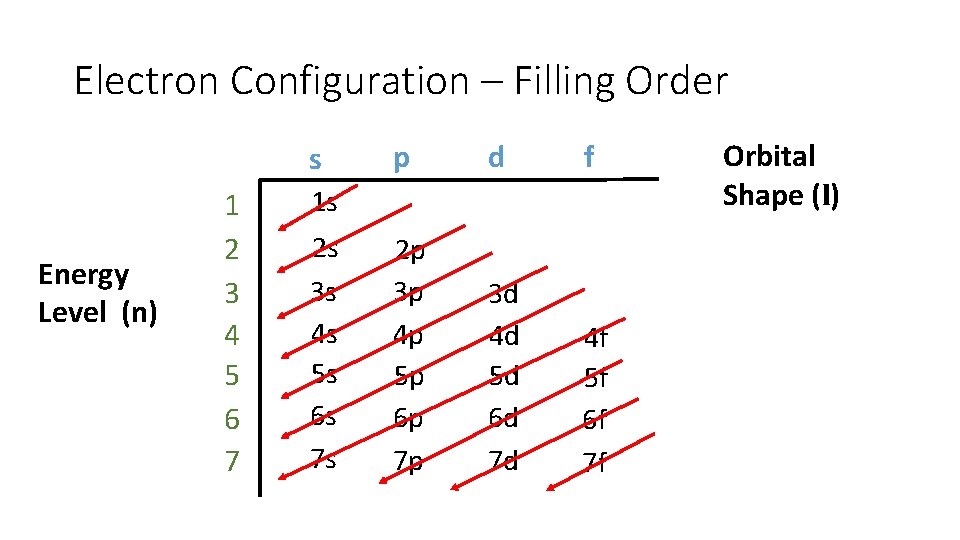
Electron Configuration – Filling Order s Energy Level (n) 1 2 3 4 5 6 7 p d f 2 p 3 p 4 p 5 p 6 p 7 p 3 d 4 d 5 d 6 d 7 d 4 f 5 f 6 f 7 f 1 s 2 s 3 s 4 s 5 s 6 s 7 s Orbital Shape (l)

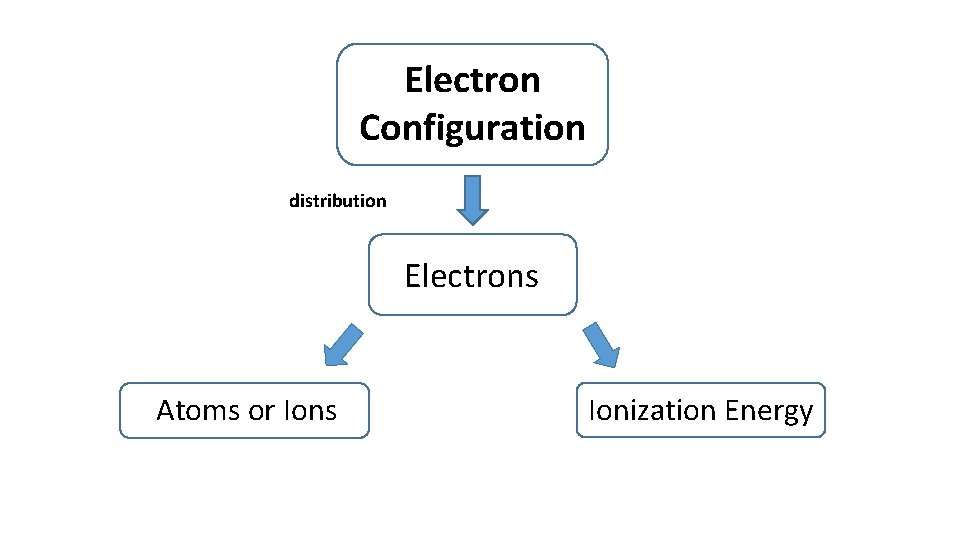
Electron Configuration distribution Electrons Atoms or Ions Ionization Energy
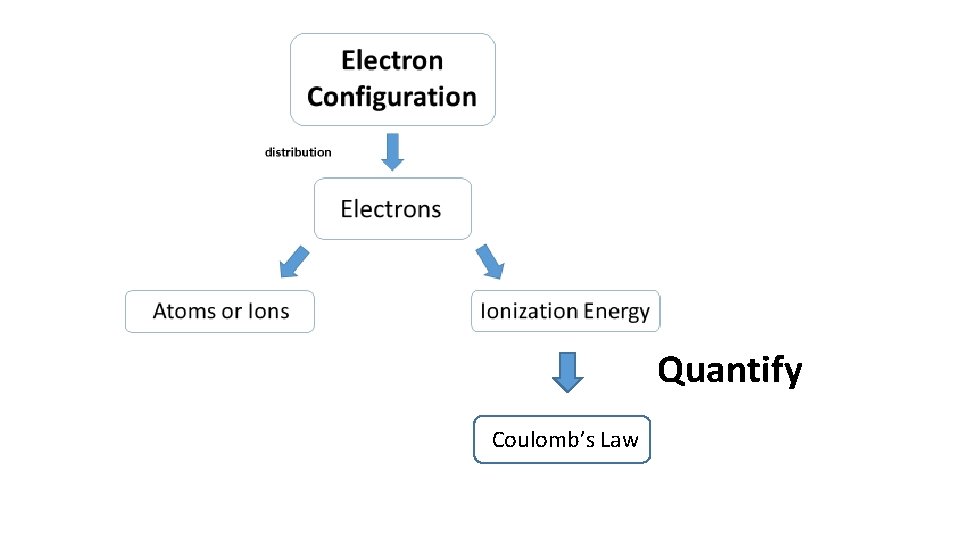
Quantify Coulomb’s Law
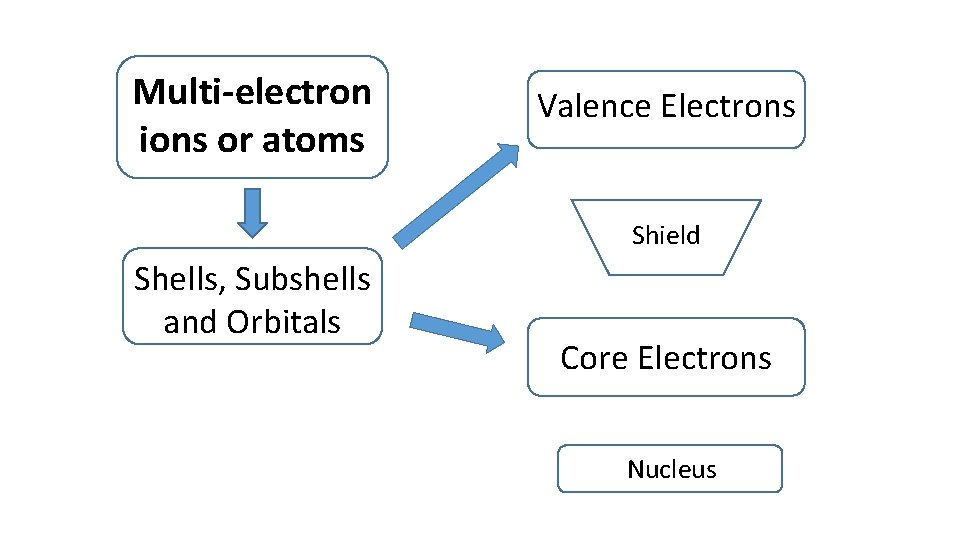
Multi-electron ions or atoms Valence Electrons Shield Shells, Subshells and Orbitals Core Electrons Nucleus
- Slides: 34