5 3 NOTES Organizing the Periodic Table Ionization

5. 3 – NOTES Organizing the Periodic Table

Ionization Energy, IE • IE definition: • energy required to remove an electron from a gaseous atom • can be thought of as the energy needed to overcome the attraction b/t the nucleus and the electron • an indication of how strongly a nucleus holds its electrons; • Equation involved: A + IE A- + e-
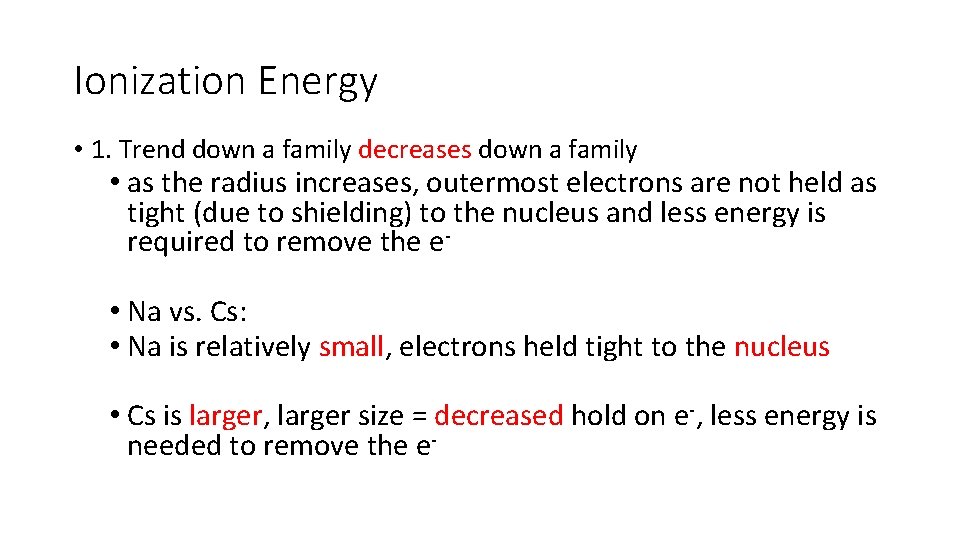
Ionization Energy • 1. Trend down a family decreases down a family • as the radius increases, outermost electrons are not held as tight (due to shielding) to the nucleus and less energy is required to remove the e • Na vs. Cs: • Na is relatively small, electrons held tight to the nucleus • Cs is larger, larger size = decreased hold on e-, less energy is needed to remove the e-

Ionization Energy • Trend across a period increases from left to right; as move , size is decreasing • • • electrons are tightly held to the nucleus (increased Zeff); Na vs. Cl: Na is larger, Cl is smaller; smaller size = tightly held e-, IE goes up; Metals have low IE’s while nonmetals have high IE’s. He has the highest IE of all elements; • In general, metals have low IE’s and nonmetals have high IE’s • The element with the highest IE is He.

Ionization Energy • Trend of successive IE’s each time an electron is removed it is known as the first ionization energy • as additional electrons are removed it becomes the 2 nd IE or the 3 rd IE • energy for each successive ionization always increases; once all valence electrons removed, the successive IE jumps drastically • Li – one valence e-, 1 st IE = 520 k. J/mol, 2 nd IE = 7300 k. J/mol • Be – 2 valence e-, 1 st IE = 900 k. J/mol, 2 nd IE = 1760 k. J/mol, 3 rd IE = 14, 850 k. J/mol

Electron Affinity, EA • E. Electron Affinity, EA • Definition – energy change that occurs when an atom gains an electron; an indication of the attraction an atom has for an electron; • Equation: A + e- A- + EA
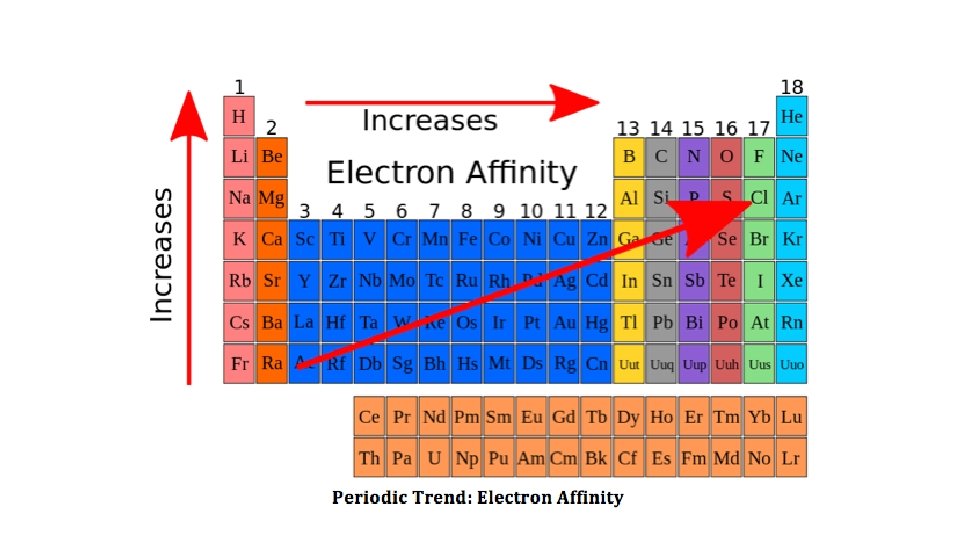
Electron Affinity • 1. Trend down a family as move down a family, EA decreases • size is increasing and larger atoms have a decreased attraction for electrons; • recall metals lose e- so gaining an e- actually adds energy to the atom; gaining energy is unfavorable • nonmetals gain e- so gaining an e- causes energy to be released; losing energy is favorable • In general, metals have low, unfavorable EA; nonmetals have high, favorable EA. • 2. Trend across a period move EA increases; atom is smaller and smaller atoms have an increased attraction to electrons, • The element with the highest EA is Cl.

Electronegativity • F. Electronegativity, EN • Definition – relative ability to attract an electron while it is bonded; ranked on a scale of 0 -4. 0 • 1. Trend down a family – decreases down a family; follows same trend as EA and IE; atoms are getting larger, the attraction for an electron decreases b/c nuclear pull would not be as strong; • 2. Trend across a period – increases as move ; same as EA and IE; atoms are getting smaller, smaller atoms hold e- tightly (nuclear pull is increasing)
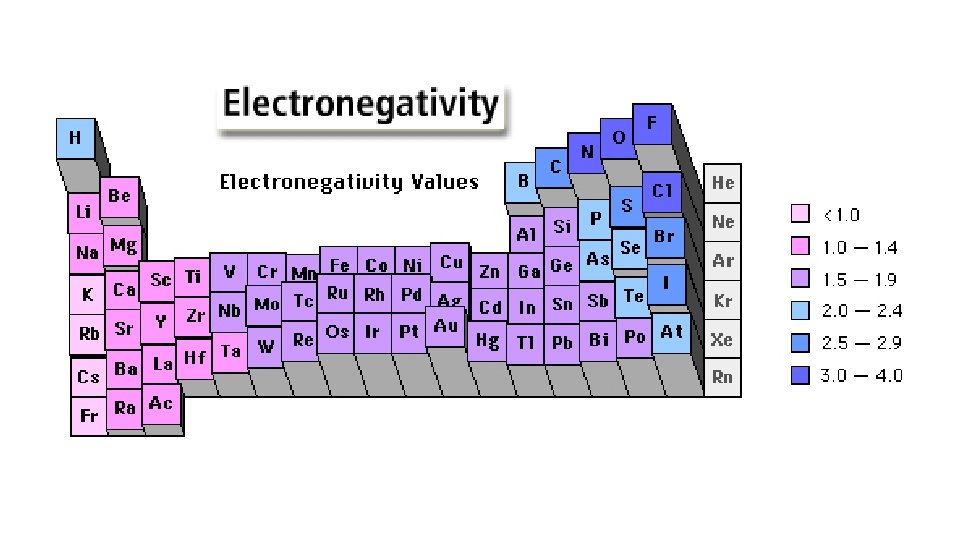
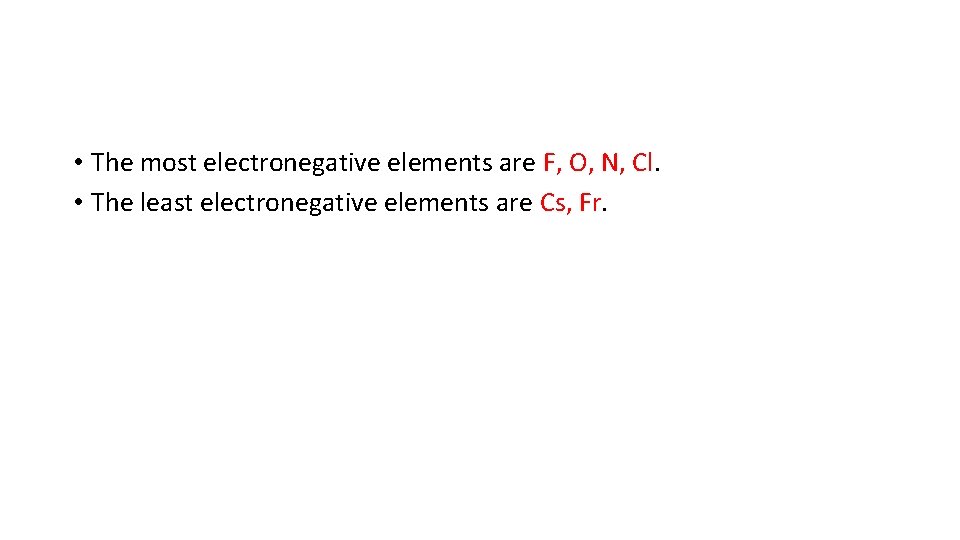
• The most electronegative elements are F, O, N, Cl. • The least electronegative elements are Cs, Fr.
- Slides: 12