5 3 Light and the Quantum Mechanical Model











- Slides: 39

5. 3 Light and the Quantum Mechanical Model Neon signs are formed from glass tubes. An electric current passing through the gas in each tube makes the gas glow with its own characteristic color. You will learn why each gas glows with a specific color of light. Slide 1 of 38 © Copyright Pearson Prentice Hall End Show

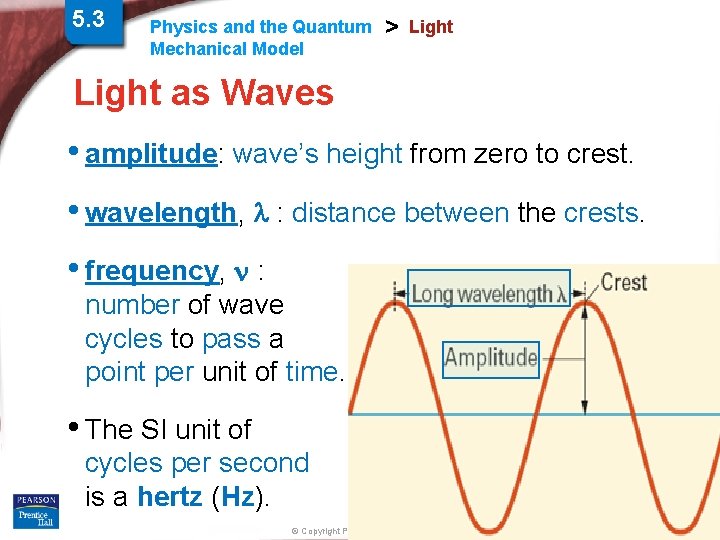
5. 3 Physics and the Quantum Mechanical Model > Light as Waves • amplitude: wave’s height from zero to crest. • wavelength, : distance between the crests. • frequency, : number of wave cycles to pass a point per unit of time. • The SI unit of cycles per second is a hertz (Hz). © Copyright Pearson Prentice Hall Slide 2 of 38 End Show

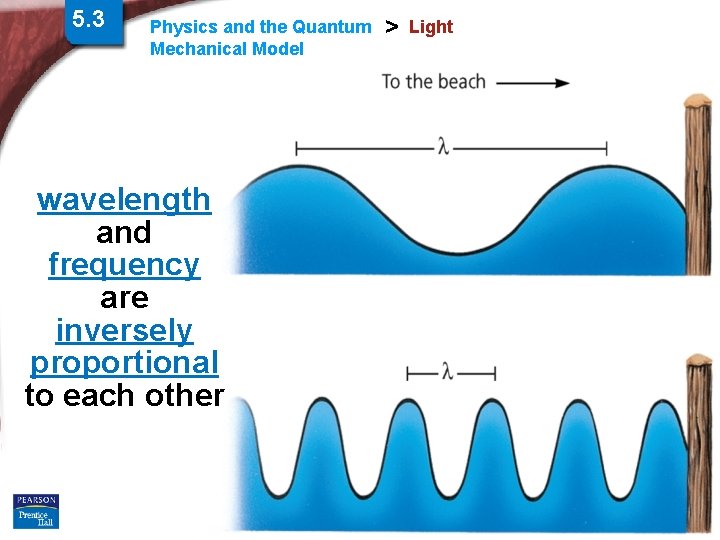
5. 3 Physics and the Quantum Mechanical Model > Light wavelength and frequency are inversely proportional to each other Slide 3 of 38 © Copyright Pearson Prentice Hall End Show

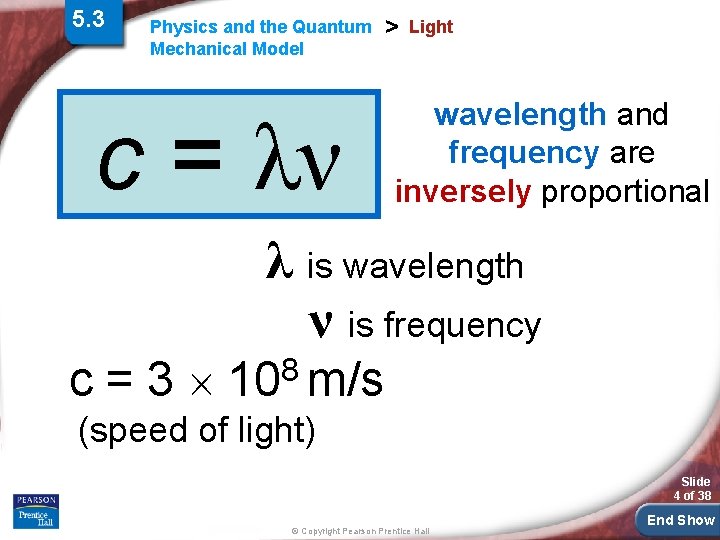
5. 3 Physics and the Quantum Mechanical Model c = λν > Light wavelength and frequency are inversely proportional λ is wavelength ν is frequency c = 3 108 m/s (speed of light) Slide 4 of 38 © Copyright Pearson Prentice Hall End Show

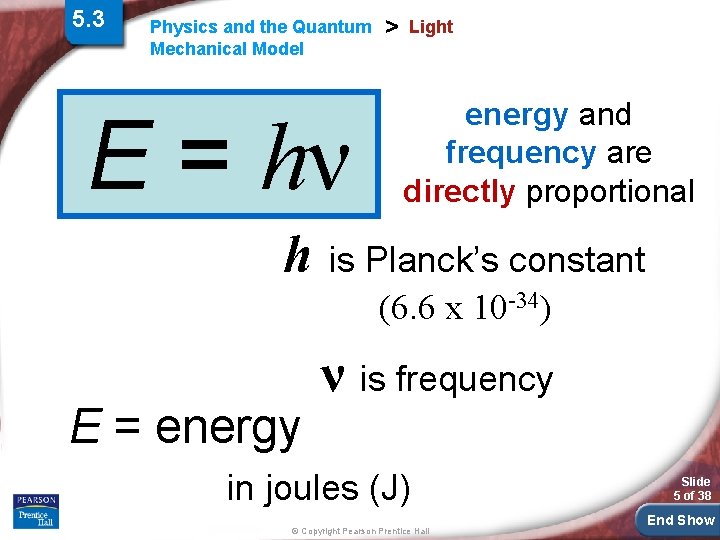
5. 3 Physics and the Quantum Mechanical Model E = hν > Light energy and frequency are directly proportional h is Planck’s constant (6. 6 x 10 -34) E = energy ν is frequency in joules (J) © Copyright Pearson Prentice Hall Slide 5 of 38 End Show

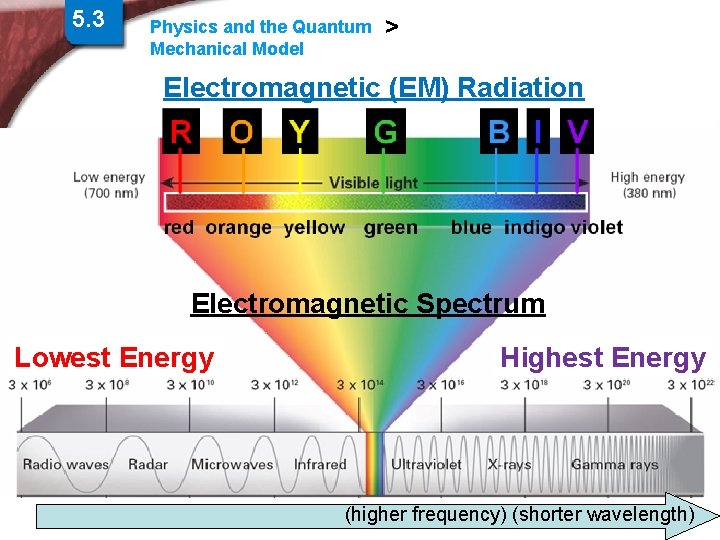
5. 3 Physics and the Quantum Mechanical Model > Electromagnetic (EM) Radiation Electromagnetic Spectrum Lowest Energy Highest Energy Slide 6 of 38 (higher frequency) (shorter wavelength) End Show © Copyright Pearson Prentice Hall

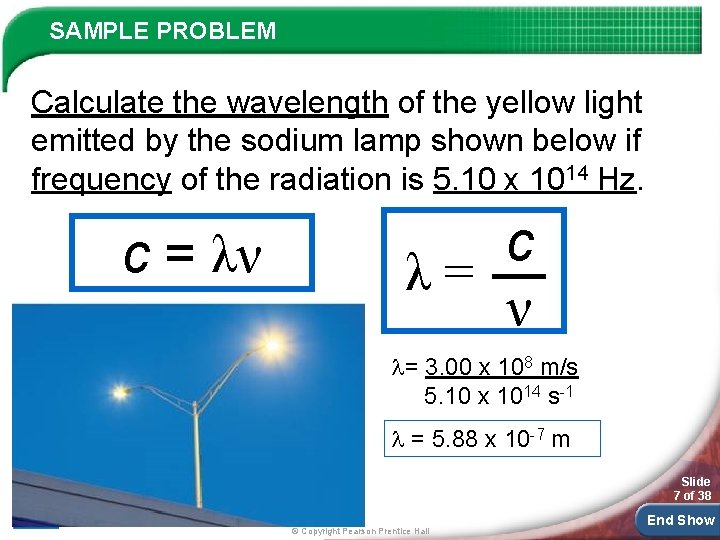
SAMPLE PROBLEM Calculate the wavelength of the yellow light emitted by the sodium lamp shown below if frequency of the radiation is 5. 10 x 1014 Hz. c = λν c λ= ν = 3. 00 x 108 m/s 5. 10 x 1014 s-1 = 5. 88 x 10 -7 m Slide 7 of 38 © Copyright Pearson Prentice Hall End Show

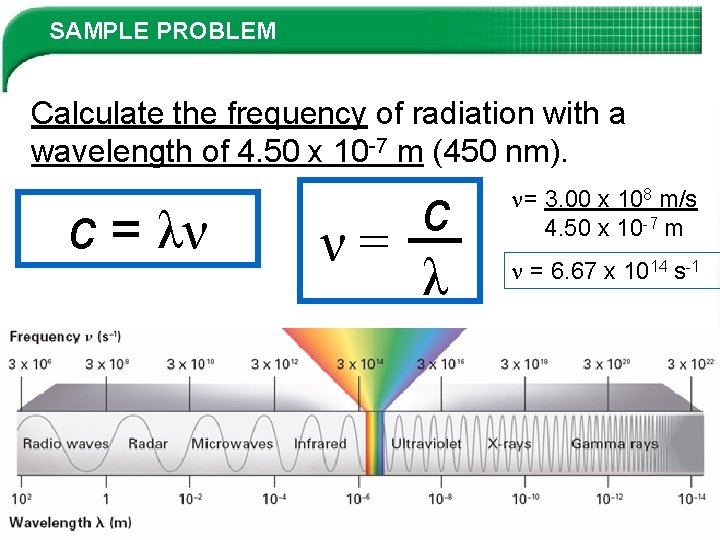
SAMPLE PROBLEM Calculate the frequency of radiation with a wavelength of 4. 50 x 10 -7 m (450 nm). c = λν c ν= λ = 3. 00 x 108 m/s 4. 50 x 10 -7 m = 6. 67 x 1014 s-1 Slide 8 of 38 © Copyright Pearson Prentice Hall End Show

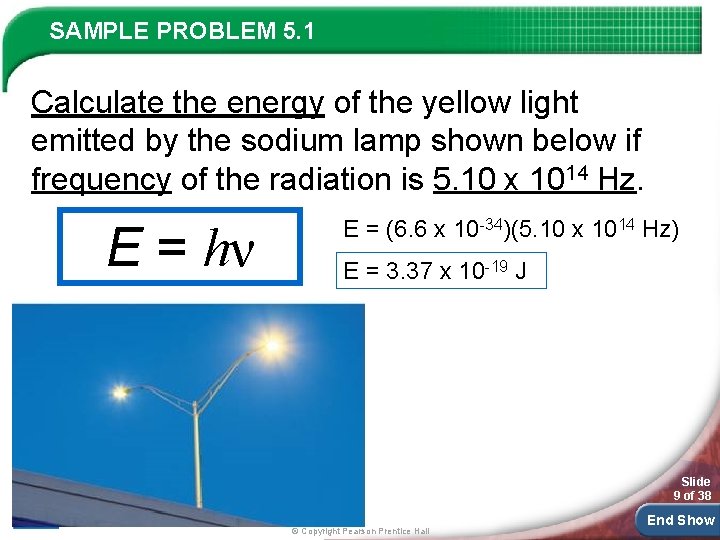
SAMPLE PROBLEM 5. 1 Calculate the energy of the yellow light emitted by the sodium lamp shown below if frequency of the radiation is 5. 10 x 1014 Hz. E = hν E = (6. 6 x 10 -34)(5. 10 x 1014 Hz) E = 3. 37 x 10 -19 J Slide 9 of 38 © Copyright Pearson Prentice Hall End Show

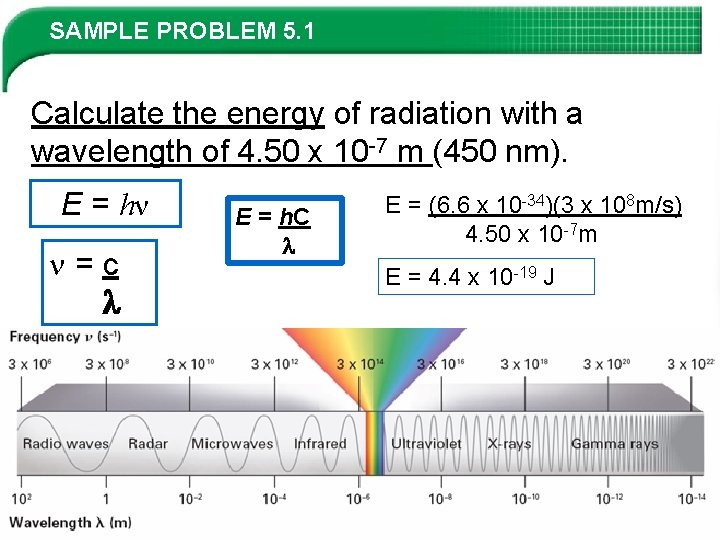
SAMPLE PROBLEM 5. 1 Calculate the energy of radiation with a wavelength of 4. 50 x 10 -7 m (450 nm). E = hν =c E = h. C E = (6. 6 x 10 -34)(3 x 108 m/s) 4. 50 x 10 -7 m E = 4. 4 x 10 -19 J Slide 10 of 38 © Copyright Pearson Prentice Hall End Show

Quick Quiz! 1. Which of the following relationships is true? A. Higher-energy light has a higher frequency than lower-energy light does. B. Higher-energy light has a longer wavelength than lower-energy light does. C. Higher-energy light travels at a faster speed than lower-energy light does. D. Higher-frequency light travels at a slower speed than lower-energy light does. © Copyright Pearson Prentice Hall Slide 11 of 38 End Show

Quick Quiz. 2. The energy of EM radiation is greatest for A. visible light. B. ultraviolet light. C. infrared light. D. X-ray radiation. Slide 12 of 38 © Copyright Pearson Prentice Hall End Show

Quick Quiz. 3. The longer the wavelength of light, the… A. higher the frequency. B. higher the energy. C. lower the energy. D. lower the frequency. Slide 13 of 38 © Copyright Pearson Prentice Hall End Show

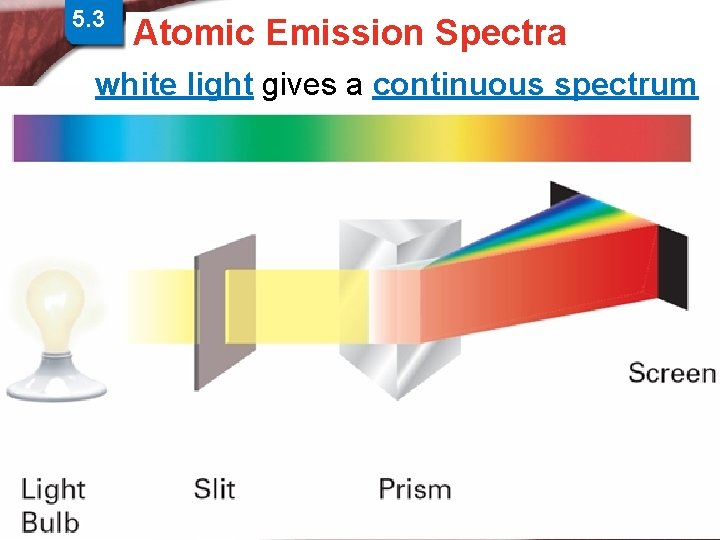
5. 3 Physics and the Quantum > Atomic Spectra Atomic Emission Spectra Mechanical Model white light gives a continuous spectrum Slide 14 of 38 © Copyright Pearson Prentice Hall End Show

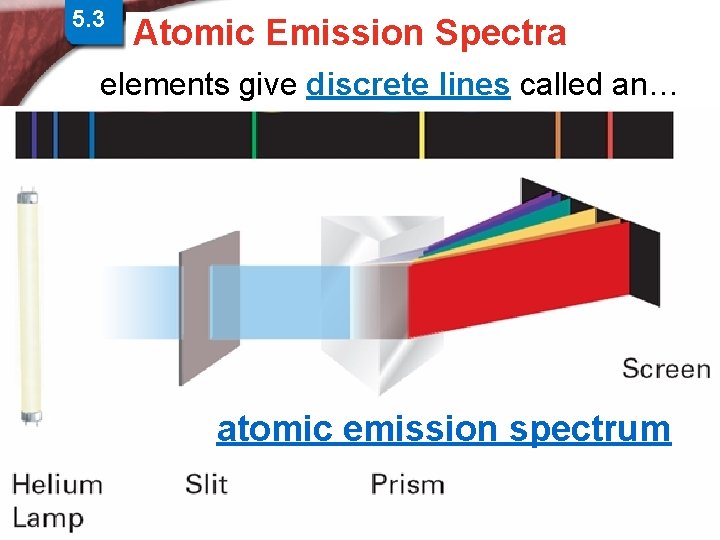
5. 3 Physics and the Quantum > Atomic Spectra Atomic Emission Spectra Mechanical Model elements give discrete lines called an… atomic emission spectrum Slide 15 of 38 © Copyright Pearson Prentice Hall End Show

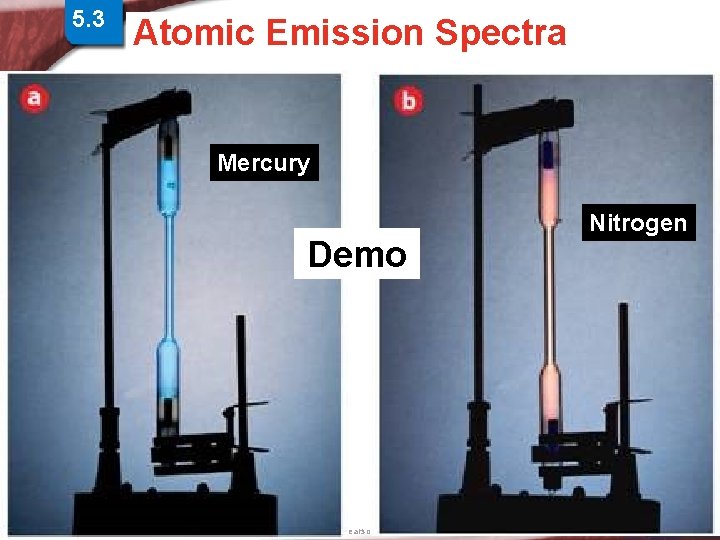
5. 3 Physics and the Quantum > Atomic Spectra Atomic Emission Spectra Mechanical Model Mercury Demo Nitrogen Slide 16 of 38 © Copyright Pearson Prentice Hall End Show

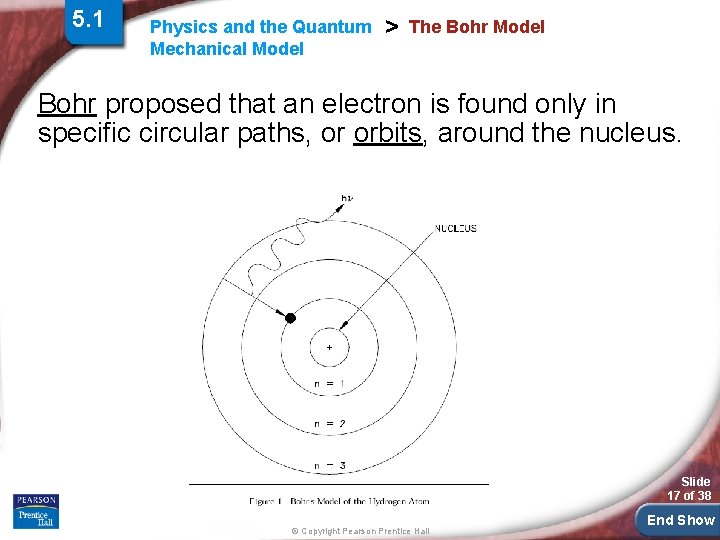
5. 1 Physics and the Quantum Mechanical Model > The Bohr Model Bohr proposed that an electron is found only in specific circular paths, or orbits, around the nucleus. Slide 17 of 38 © Copyright Pearson Prentice Hall End Show

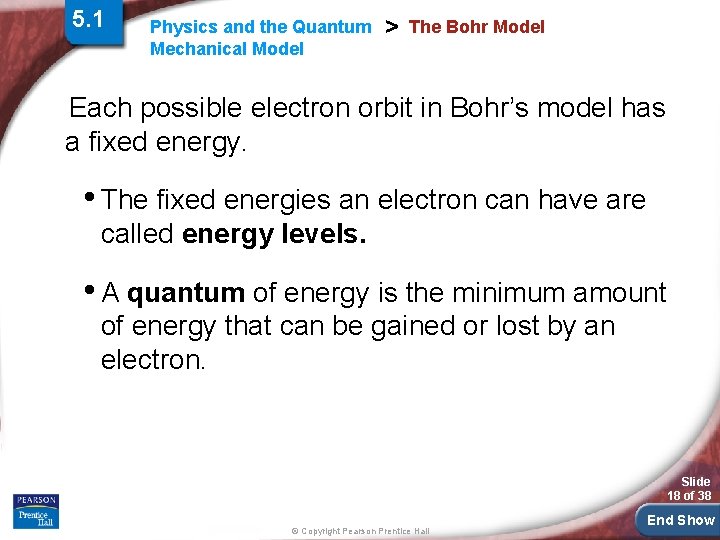
5. 1 Physics and the Quantum Mechanical Model > The Bohr Model Each possible electron orbit in Bohr’s model has a fixed energy. • The fixed energies an electron can have are called energy levels. • A quantum of energy is the minimum amount of energy that can be gained or lost by an electron. Slide 18 of 38 © Copyright Pearson Prentice Hall End Show

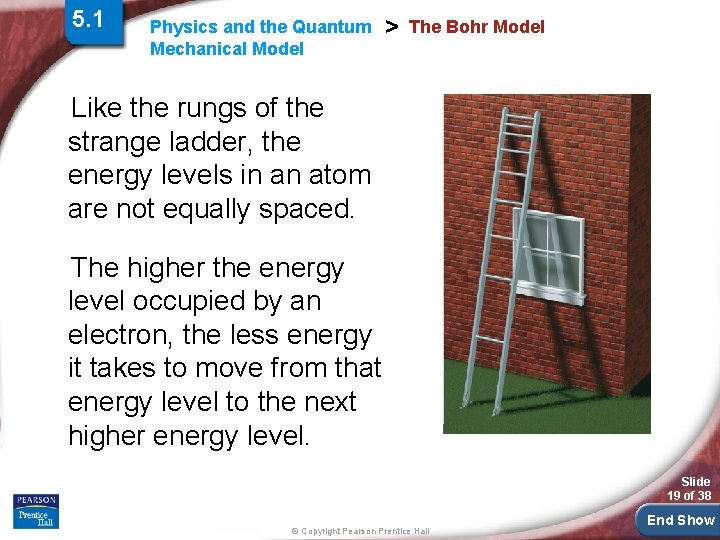
5. 1 Physics and the Quantum Mechanical Model > The Bohr Model Like the rungs of the strange ladder, the energy levels in an atom are not equally spaced. The higher the energy level occupied by an electron, the less energy it takes to move from that energy level to the next higher energy level. Slide 19 of 38 © Copyright Pearson Prentice Hall End Show

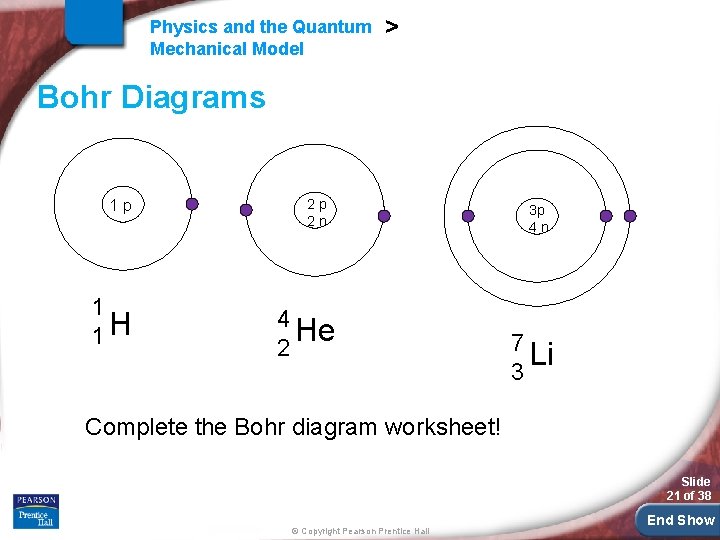
Physics and the Quantum Mechanical Model > Bohr Diagrams 2, 8, 8 rule - up to 2 electrons can fit in the first energy level - up to 8 electrons can fit in the second energy level - up to 8 electrons can fit in the third energy level How many energy levels? - The number of energy levels corresponds to the period number (row number) of the element Ex – H has one energy level; Li has two; Na has three, etc. Slide 20 of 38 © Copyright Pearson Prentice Hall End Show

Physics and the Quantum Mechanical Model > Bohr Diagrams 1 p 1 1 H 2 p 2 n 4 He 2 3 p 4 n 7 Li 3 Complete the Bohr diagram worksheet! Slide 21 of 38 © Copyright Pearson Prentice Hall End Show

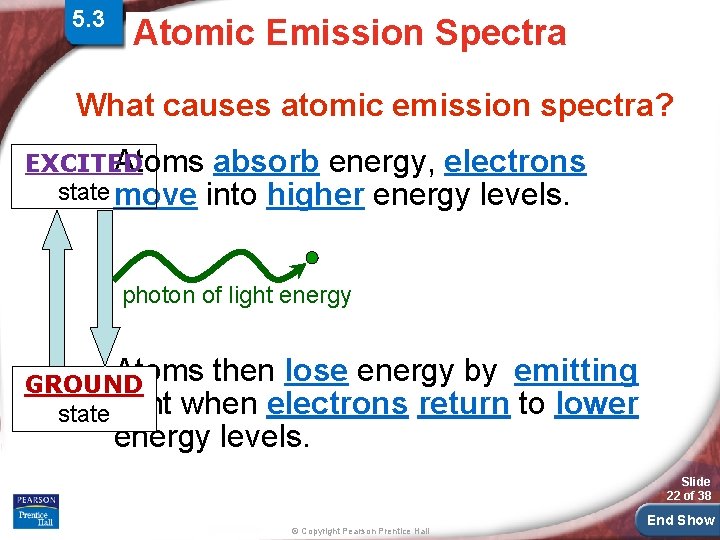
5. 3 Physics and the Quantum > Atomic Spectra Atomic Emission Spectra Mechanical Model What causes atomic emission spectra? EXCITED Atoms absorb energy, electrons state move into higher energy levels. photon of light energy Atoms then lose energy by emitting when electrons return to lower energy levels. GROUND state light Slide 22 of 38 © Copyright Pearson Prentice Hall End Show

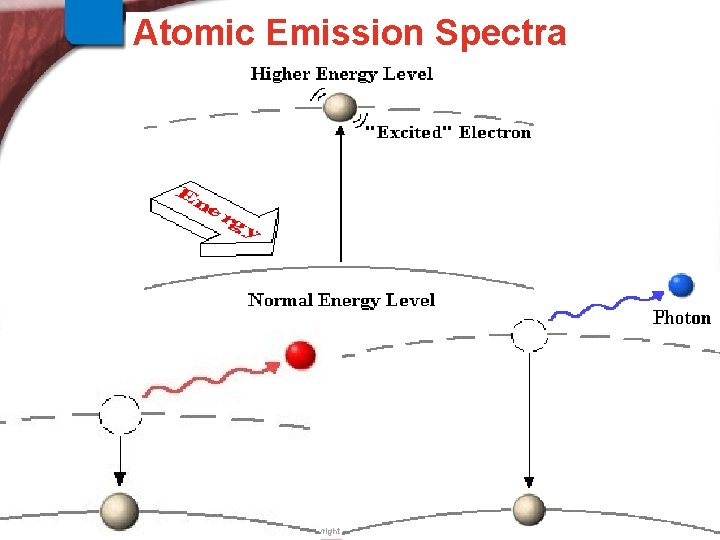
Physics and the Quantum > Atomic Emission Spectra Mechanical Model Slide 23 of 38 © Copyright Pearson Prentice Hall End Show

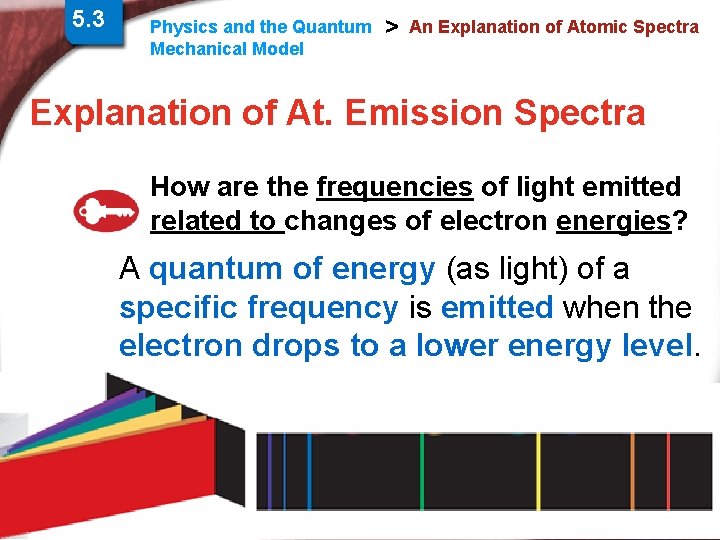
5. 3 Physics and the Quantum Mechanical Model > An Explanation of Atomic Spectra Explanation of At. Emission Spectra How are the frequencies of light emitted related to changes of electron energies? A quantum of energy (as light) of a specific frequency is emitted when the electron drops to a lower energy level. Slide 24 of 38 © Copyright Pearson Prentice Hall End Show

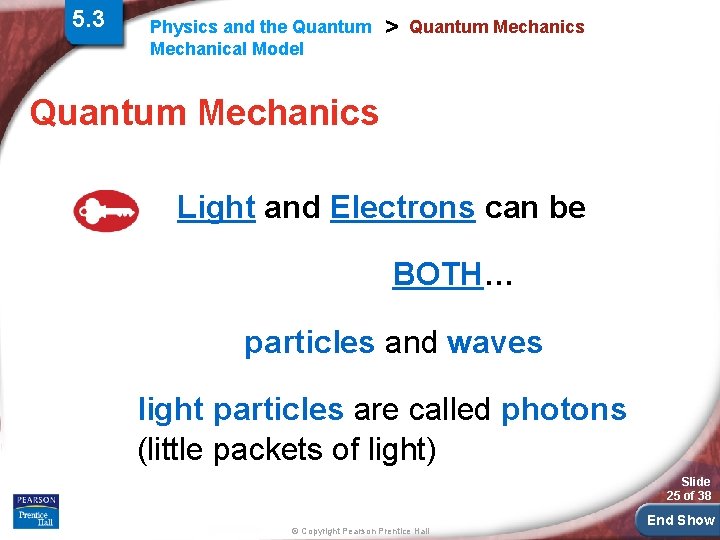
5. 3 Physics and the Quantum Mechanical Model > Quantum Mechanics Light and Electrons can be BOTH… particles and waves light particles are called photons (little packets of light) Slide 25 of 38 © Copyright Pearson Prentice Hall End Show

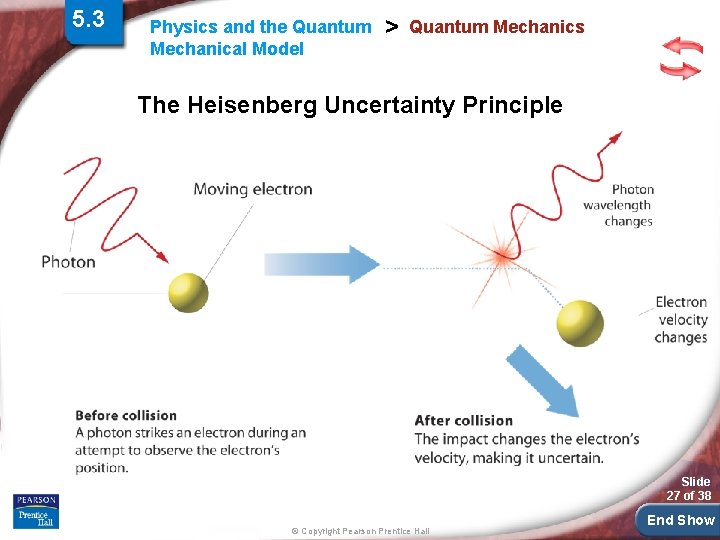
5. 3 Physics and the Quantum Mechanical Model > Quantum Mechanics Heisenberg uncertainty principle cannot know exactly both the velocity and the position of a particle at the same time. • only critical with small particles like electrons & photons. (not cars and airplanes) Slide 26 of 38 © Copyright Pearson Prentice Hall End Show

5. 3 Physics and the Quantum Mechanical Model > Quantum Mechanics The Heisenberg Uncertainty Principle Slide 27 of 38 © Copyright Pearson Prentice Hall End Show

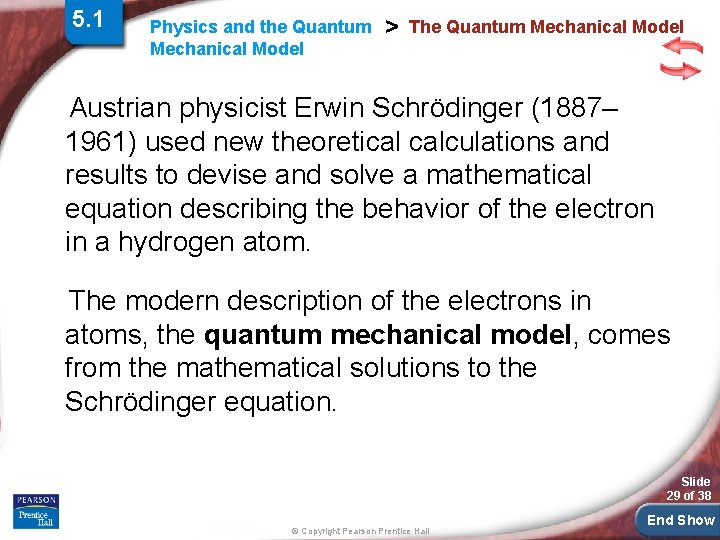
5. 1 Physics and the Quantum Mechanical Model > The Quantum Mechanical Model The quantum mechanical model determines the allowed energies an electron can have and how likely it is to find the electron in various locations around the nucleus. Slide 28 of 38 © Copyright Pearson Prentice Hall End Show

5. 1 Physics and the Quantum Mechanical Model > The Quantum Mechanical Model Austrian physicist Erwin Schrödinger (1887– 1961) used new theoretical calculations and results to devise and solve a mathematical equation describing the behavior of the electron in a hydrogen atom. The modern description of the electrons in atoms, the quantum mechanical model, comes from the mathematical solutions to the Schrödinger equation. Slide 29 of 38 © Copyright Pearson Prentice Hall End Show

5. 1 Physics and the Quantum Mechanical Model > The Quantum Mechanical Model The propeller blade has the same probability of being anywhere in the blurry region, but you cannot tell its location at any instant. The electron cloud of an atom can be compared to a spinning airplane propeller. Slide 30 of 38 © Copyright Pearson Prentice Hall End Show

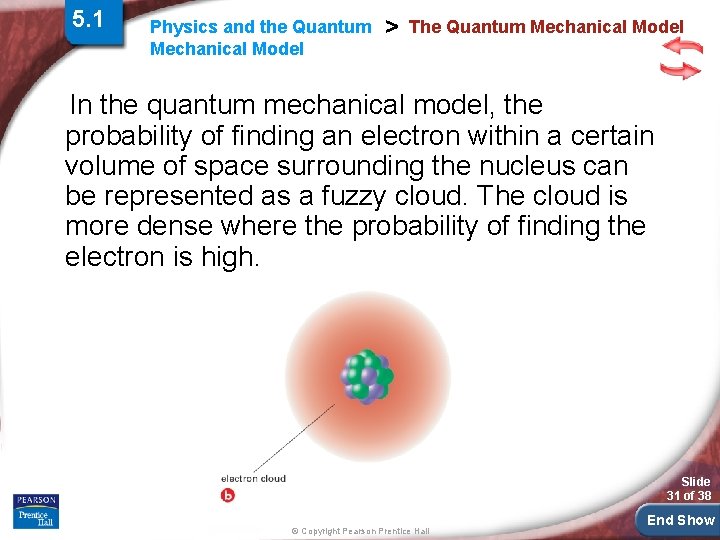
5. 1 Physics and the Quantum Mechanical Model > The Quantum Mechanical Model In the quantum mechanical model, the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. The cloud is more dense where the probability of finding the electron is high. Slide 31 of 38 © Copyright Pearson Prentice Hall End Show

5. 1 Physics and the Quantum Mechanical Model > Atomic Orbitals An atomic orbital is often thought of as a region of space in which there is a high probability of finding an electron. Each energy sublevel corresponds to an orbital of a different shape, which describes where the electron is likely to be found. Slide 32 of 38 © Copyright Pearson Prentice Hall End Show

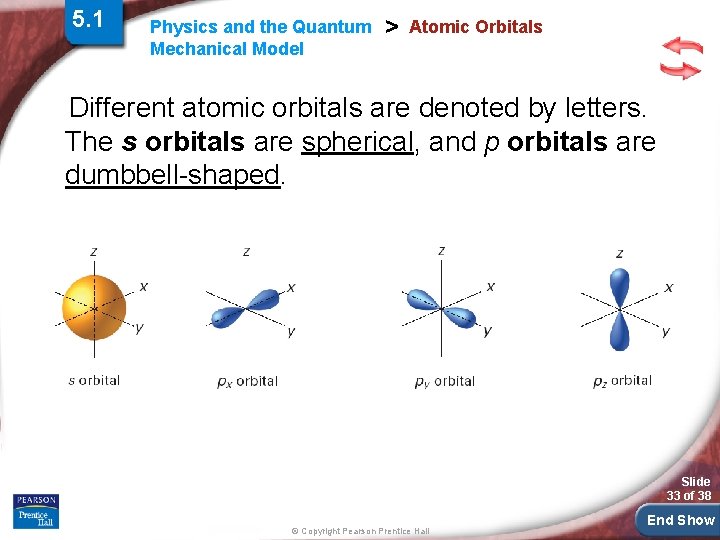
5. 1 Physics and the Quantum Mechanical Model > Atomic Orbitals Different atomic orbitals are denoted by letters. The s orbitals are spherical, and p orbitals are dumbbell-shaped. Slide 33 of 38 © Copyright Pearson Prentice Hall End Show

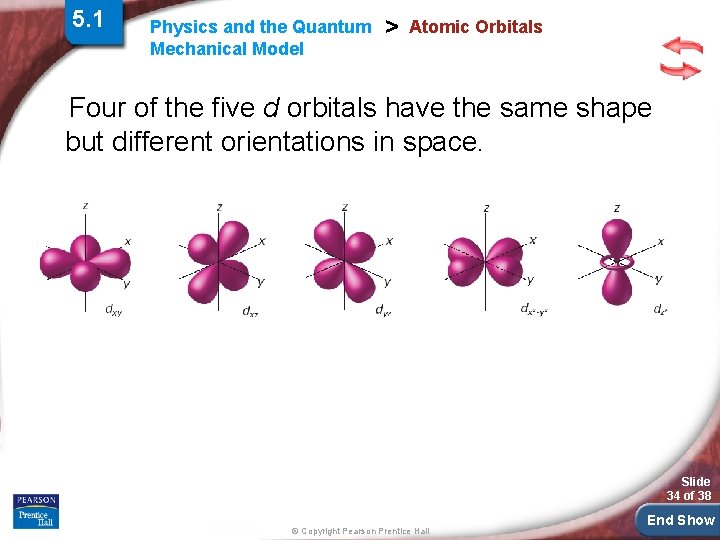
5. 1 Physics and the Quantum Mechanical Model > Atomic Orbitals Four of the five d orbitals have the same shape but different orientations in space. Slide 34 of 38 © Copyright Pearson Prentice Hall End Show

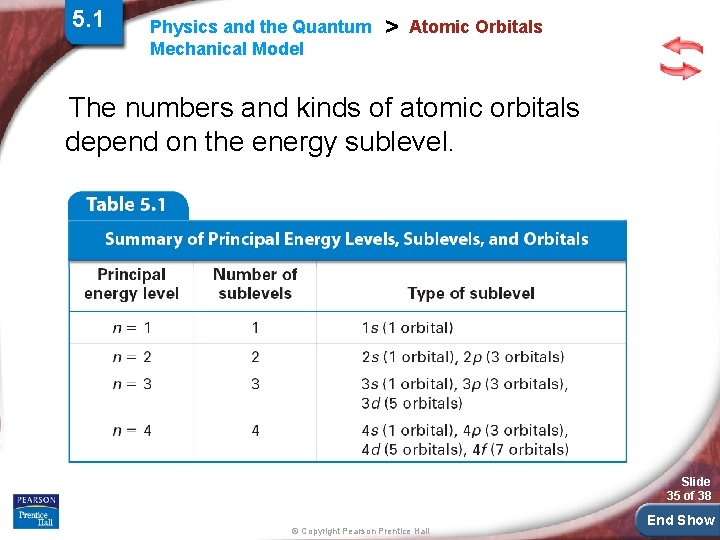
5. 1 Physics and the Quantum Mechanical Model > Atomic Orbitals The numbers and kinds of atomic orbitals depend on the energy sublevel. Slide 35 of 38 © Copyright Pearson Prentice Hall End Show

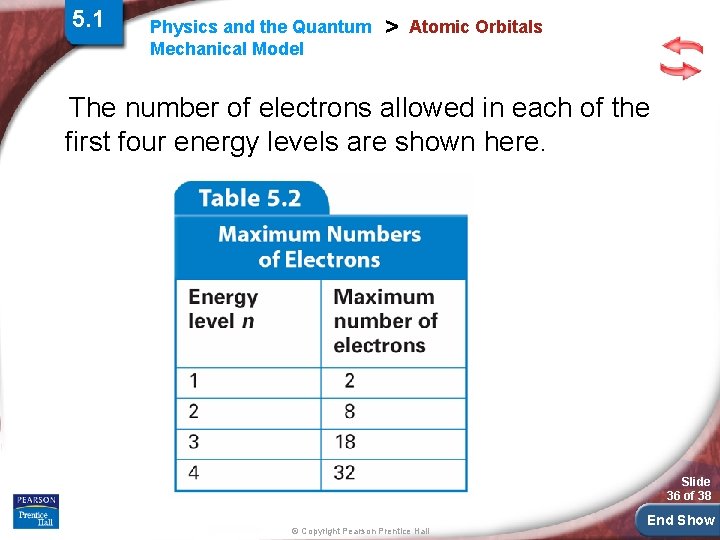
5. 1 Physics and the Quantum Mechanical Model > Atomic Orbitals The number of electrons allowed in each of the first four energy levels are shown here. Slide 36 of 38 © Copyright Pearson Prentice Hall End Show

Quick Quiz! 1. The lines in the emission spectrum for an element are caused by A. the movement of electrons from lower up to higher energy levels. B. the movement of electrons from higher down to lower energy levels. C. the electron configuration in the ground state. D. the electron configuration of an atom. © Copyright Pearson Prentice Hall Slide 37 of 38 End Show

Quick Quiz. 2. Which transition in an excited hydrogen atom will emit the longest wavelength of light? A. E 5 to E 3 B. E 4 to E 1 C. E 3 to E 2 D. highest energy/frequency Slide 38 of 38 E 3 to E 1 © Copyright Pearson Prentice Hall End Show

Quick Quiz. 3. According to the Heisenberg uncertainty principle, if the velocity of a particle is known, what other quantity CANNOT be known? A. mass B. charge C. position D. spin Slide 39 of 38 © Copyright Pearson Prentice Hall End Show