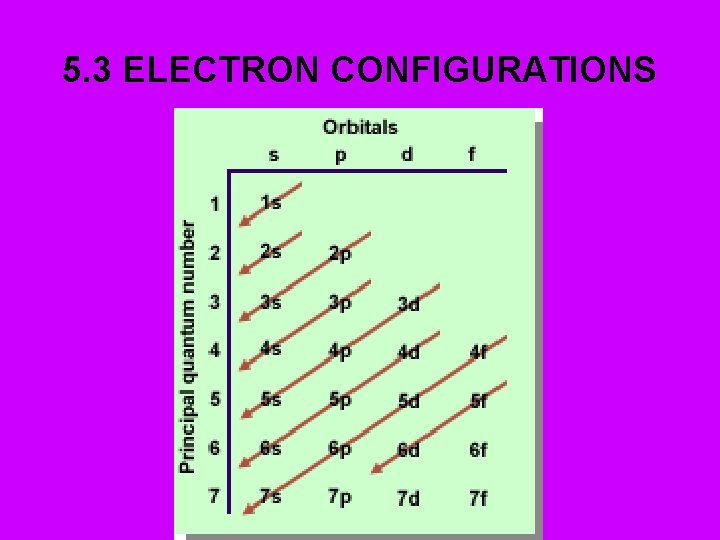
5 3 ELECTRON CONFIGURATIONS 5 3 ELECTRON CONFIGURATION




![EXCEPTIONS (cont. ) • Cu #29 • Predicted configuration: • [Ar] 4 s 2 EXCEPTIONS (cont. ) • Cu #29 • Predicted configuration: • [Ar] 4 s 2](https://slidetodoc.com/presentation_image_h2/69e4d608d0278ffa99882b36141544a9/image-26.jpg)
- Slides: 29

5. 3 ELECTRON CONFIGURATIONS

5. 3 ELECTRON CONFIGURATION Arrangement of electrons in the atom: • Electron Hotel: • 1) Stays in the cheapest • (lowest energy) room possible. • 2) No more than 2 (electrons) to a room. • 3) Likes having own room. Will share if next room is more expensive. • 4) “d” & “f” suites are especially expensive!

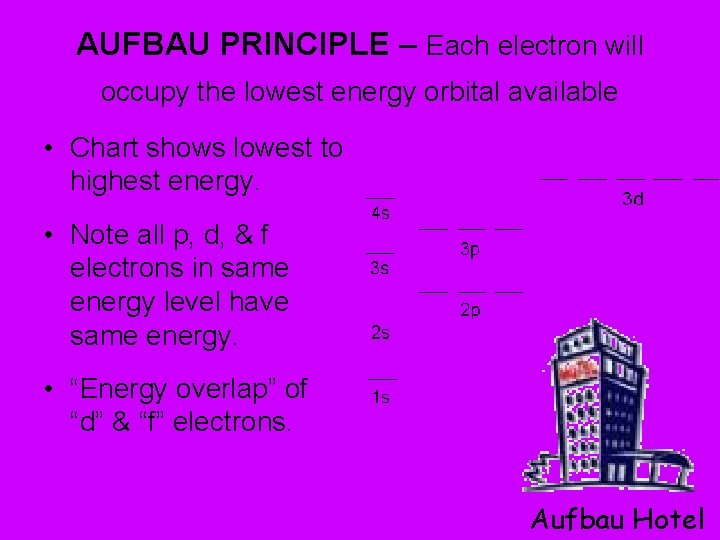
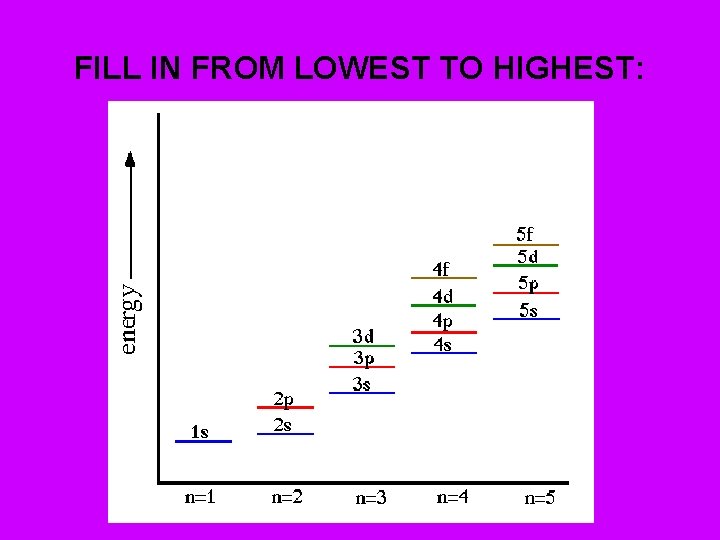
AUFBAU PRINCIPLE – Each electron will occupy the lowest energy orbital available • Chart shows lowest to highest energy. • Note all p, d, & f electrons in same energy level have same energy. • “Energy overlap” of “d” & “f” electrons. Aufbau Hotel

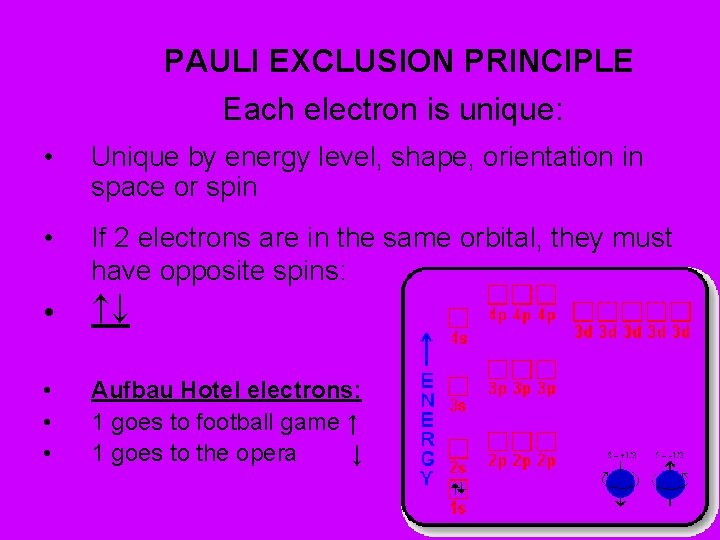
PAULI EXCLUSION PRINCIPLE Each electron is unique: • Unique by energy level, shape, orientation in space or spin • If 2 electrons are in the same orbital, they must have opposite spins: • • Aufbau Hotel electrons: 1 goes to football game ↑ 1 goes to the opera ↓

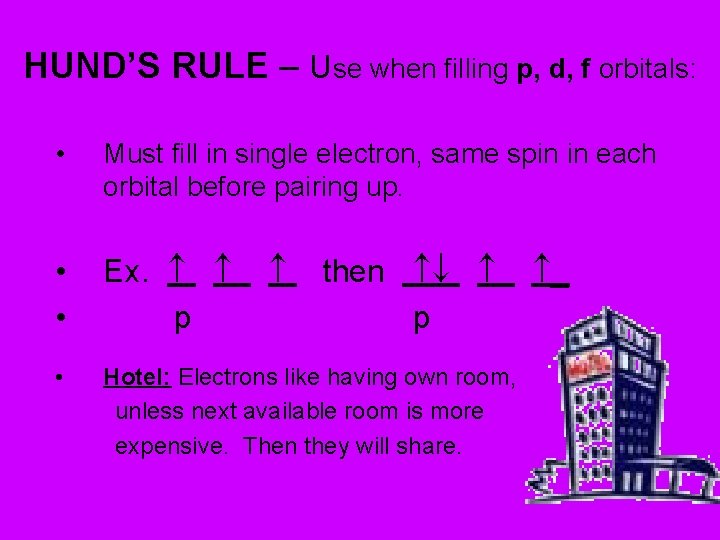
HUND’S RULE – Use when filling p, d, f orbitals: • Must fill in single electron, same spin in each orbital before pairing up. • • Ex. p • Hotel: Electrons like having own room, unless next available room is more expensive. Then they will share. then p _

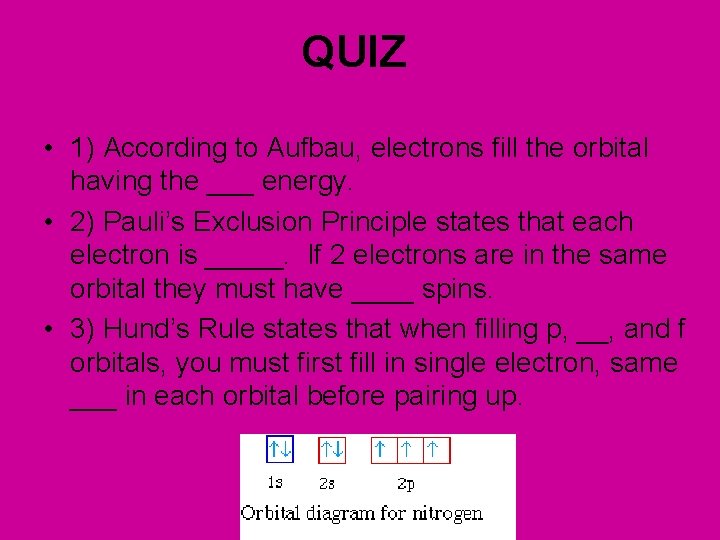
QUIZ • 1) According to Aufbau, electrons fill the orbital having the ___ energy. • 2) Pauli’s Exclusion Principle states that each electron is _____. If 2 electrons are in the same orbital they must have ____ spins. • 3) Hund’s Rule states that when filling p, __, and f orbitals, you must first fill in single electron, same ___ in each orbital before pairing up.

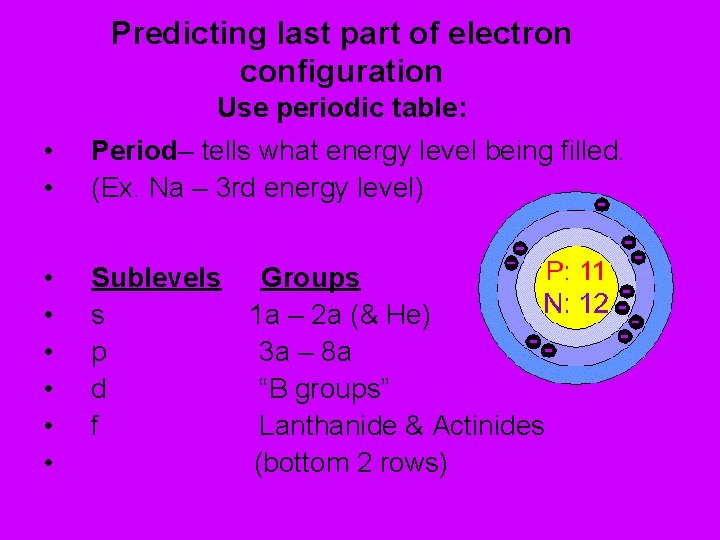
Predicting last part of electron configuration Use periodic table: • • Period– tells what energy level being filled. (Ex. Na – 3 rd energy level) • • • Sublevels Groups s 1 a – 2 a (& He) p 3 a – 8 a d “B groups” f Lanthanide & Actinides (bottom 2 rows)

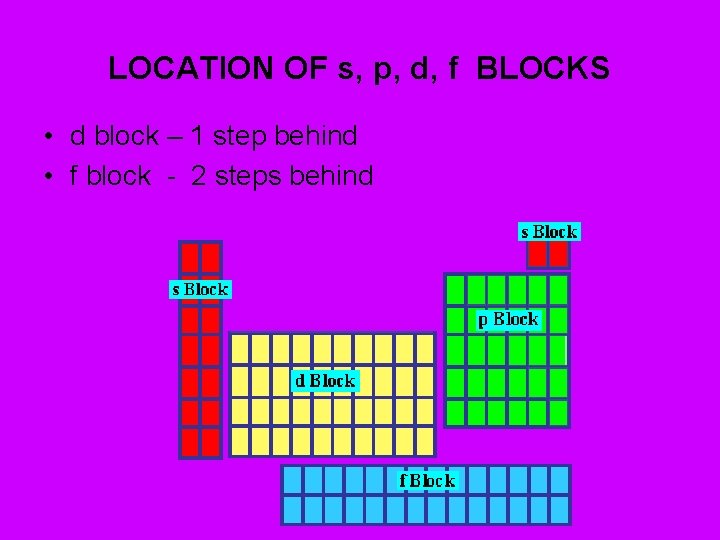
LOCATION OF s, p, d, f BLOCKS • d block – 1 step behind • f block - 2 steps behind

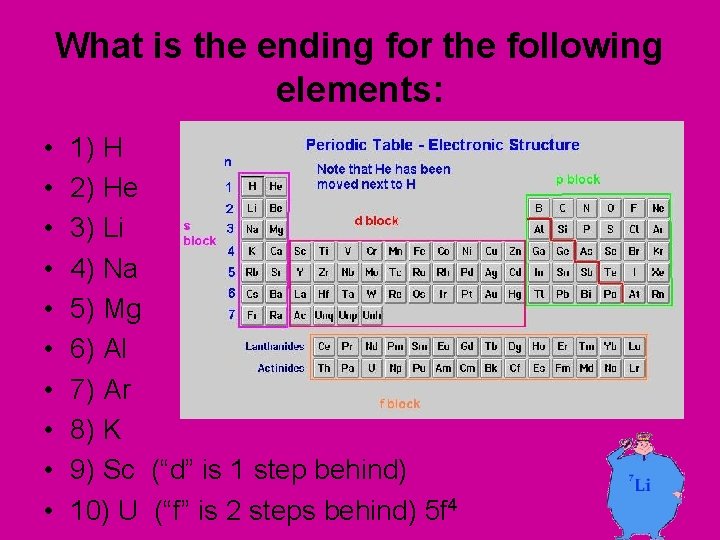
What is the ending for the following elements: • • • 1) H 2) He 3) Li 4) Na 5) Mg 6) Al 7) Ar 8) K 9) Sc (“d” is 1 step behind) 10) U (“f” is 2 steps behind) 5 f 4

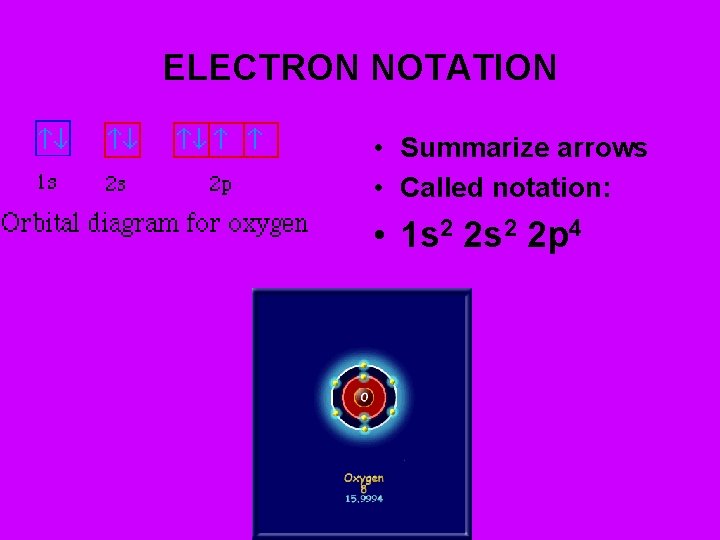
WRITING ORBITAL DIAGRAMS: Ex. O • 1) Find atomic #. Gives #electrons (arrows). • For O, atomic # = 8 • 2) Find what O ends in. • 2 p 4. • 3) Draw orbitals (slots) from lowest (1 s) to 2 p 4. • __ __ __ Now draw in arrows. • 1 s 2 s 2 p • Use Hotel rules:

FILL IN FROM LOWEST TO HIGHEST:

ELECTRON NOTATION • Summarize arrows • Called notation: • 1 s 2 2 p 4

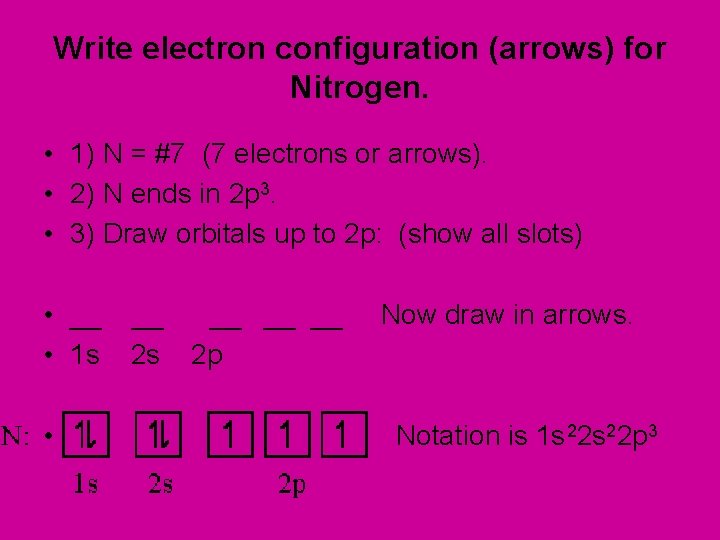
Write electron configuration (arrows) for Nitrogen. • 1) N = #7 (7 electrons or arrows). • 2) N ends in 2 p 3. • 3) Draw orbitals up to 2 p: (show all slots) • __ • 1 s • __ __ 2 s 2 p Now draw in arrows. Notation is 1 s 22 p 3

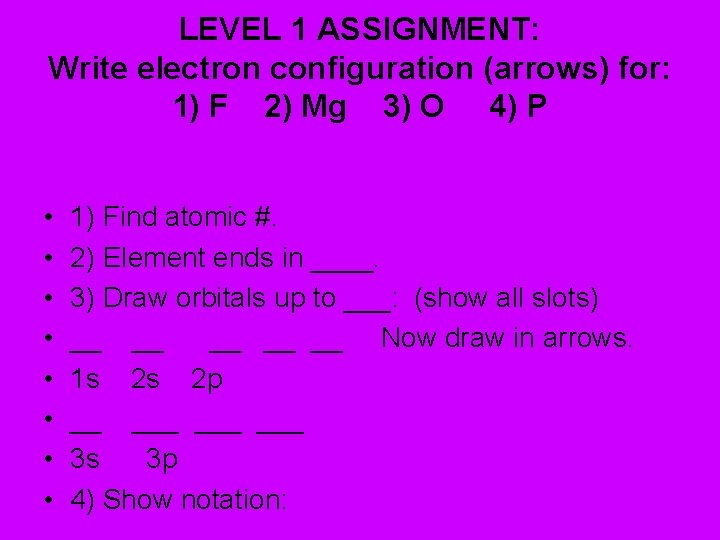
LEVEL 1 ASSIGNMENT: Write electron configuration (arrows) for: 1) F 2) Mg 3) O 4) P • • 1) Find atomic #. 2) Element ends in ____. 3) Draw orbitals up to ___: (show all slots) __ __ __ Now draw in arrows. 1 s 2 s 2 p __ ___ ___ 3 s 3 p 4) Show notation:

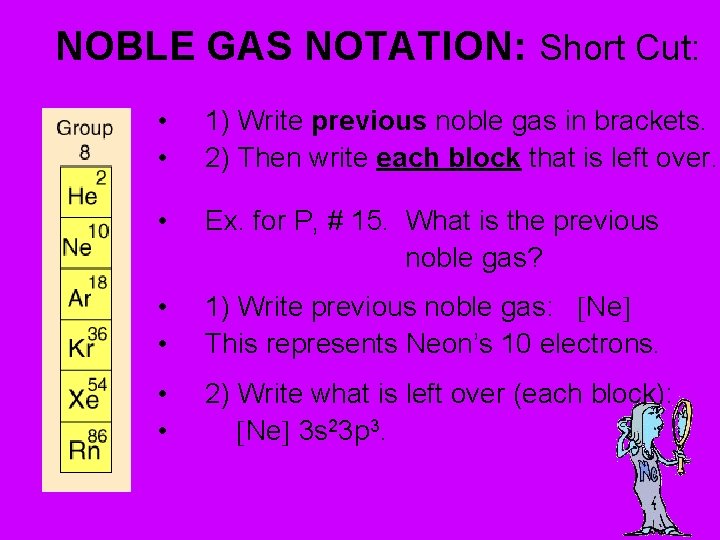
NOBLE GAS NOTATION: Short Cut: • • 1) Write previous noble gas in brackets. 2) Then write each block that is left over. • Ex. for P, # 15. What is the previous noble gas? • • 1) Write previous noble gas: Ne This represents Neon’s 10 electrons. • • 2) Write what is left over (each block): Ne 3 s 23 p 3.

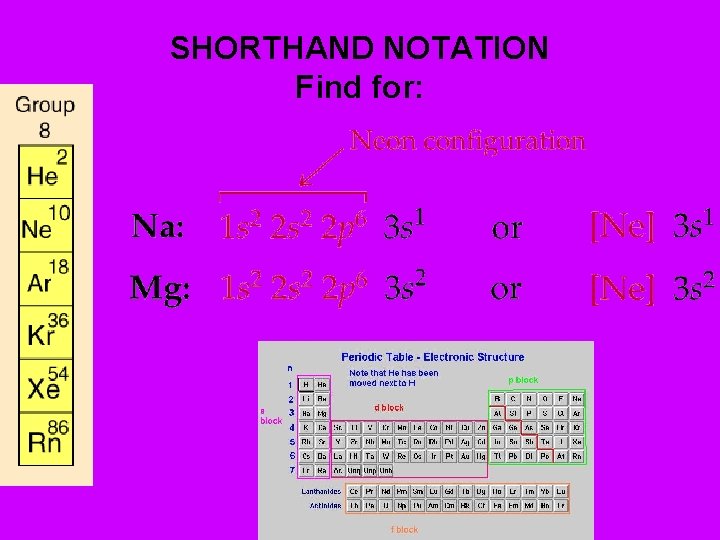
SHORTHAND NOTATION Find for:

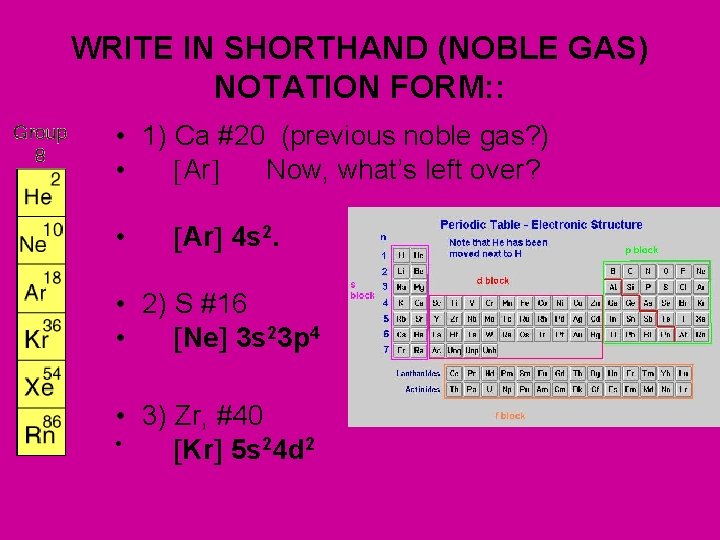
WRITE IN SHORTHAND (NOBLE GAS) NOTATION FORM: : • 1) Ca #20 (previous noble gas? ) • Ar Now, what’s left over? • Ar 4 s 2. • 2) S #16 • Ne 3 s 23 p 4 • 3) Zr, #40 • Kr 5 s 24 d 2

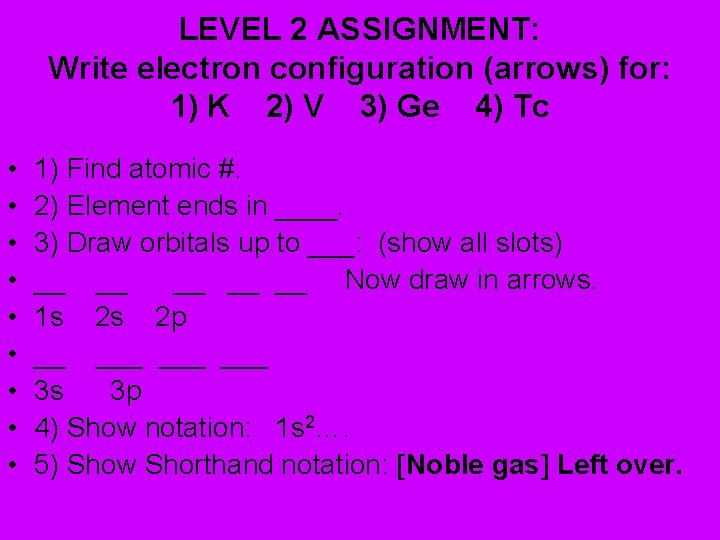
LEVEL 2 ASSIGNMENT: Write electron configuration (arrows) for: 1) K 2) V 3) Ge 4) Tc • • • 1) Find atomic #. 2) Element ends in ____. 3) Draw orbitals up to ___: (show all slots) __ __ __ Now draw in arrows. 1 s 2 s 2 p __ ___ ___ 3 s 3 p 4) Show notation: 1 s 2…. 5) Show Shorthand notation: Noble gas Left over.

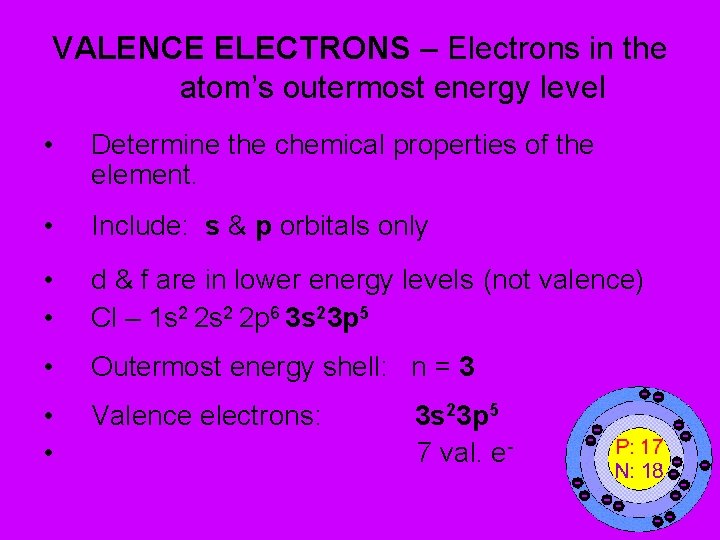
VALENCE ELECTRONS – Electrons in the atom’s outermost energy level • Determine the chemical properties of the element. • Include: s & p orbitals only • • d & f are in lower energy levels (not valence) Cl – 1 s 2 2 p 6 3 s 23 p 5 • Outermost energy shell: n = 3 • • Valence electrons: 3 s 23 p 5 7 val. e -

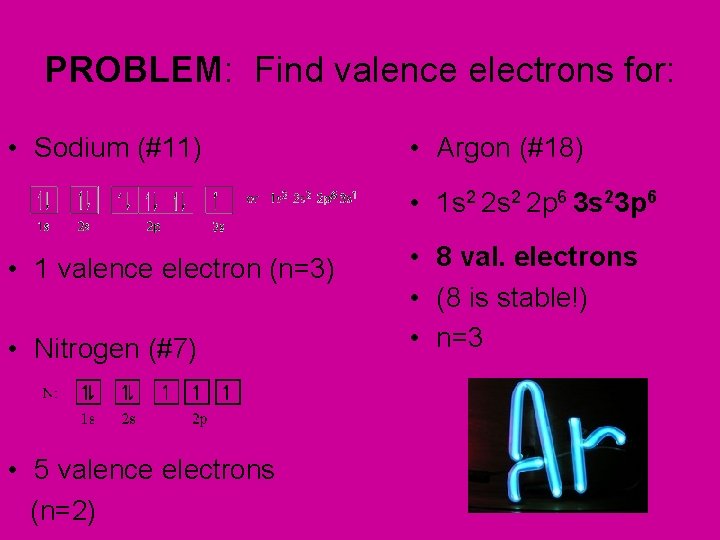
PROBLEM: Find valence electrons for: • Sodium (#11) • Argon (#18) • 1 s 2 2 p 6 3 s 23 p 6 • 1 valence electron (n=3) • Nitrogen (#7) • 5 valence electrons (n=2) • 8 val. electrons • (8 is stable!) • n=3

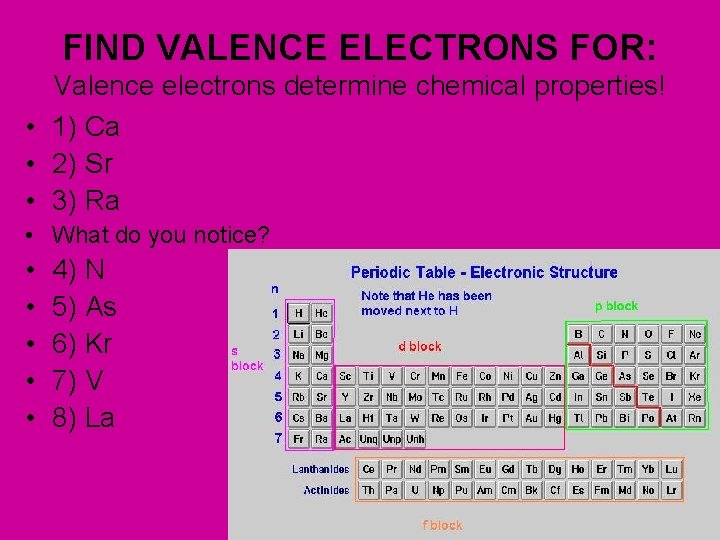
FIND VALENCE ELECTRONS FOR: Valence electrons determine chemical properties! • 1) Ca • 2) Sr • 3) Ra • What do you notice? • • • 4) N 5) As 6) Kr 7) V 8) La

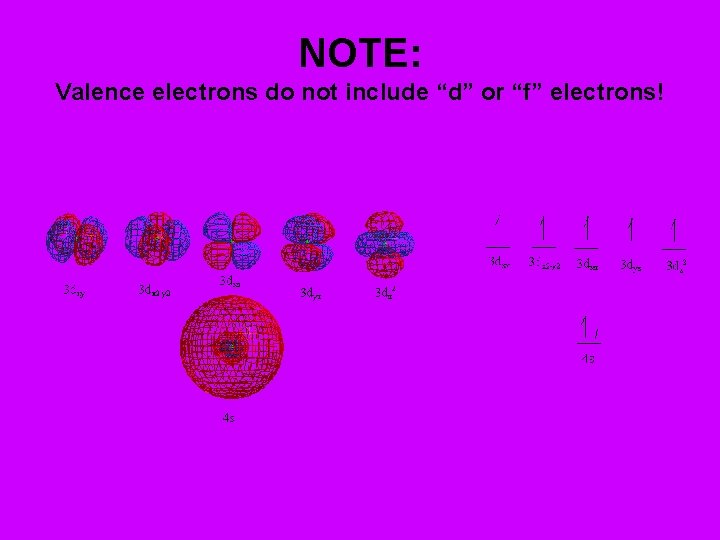
NOTE: Valence electrons do not include “d” or “f” electrons!

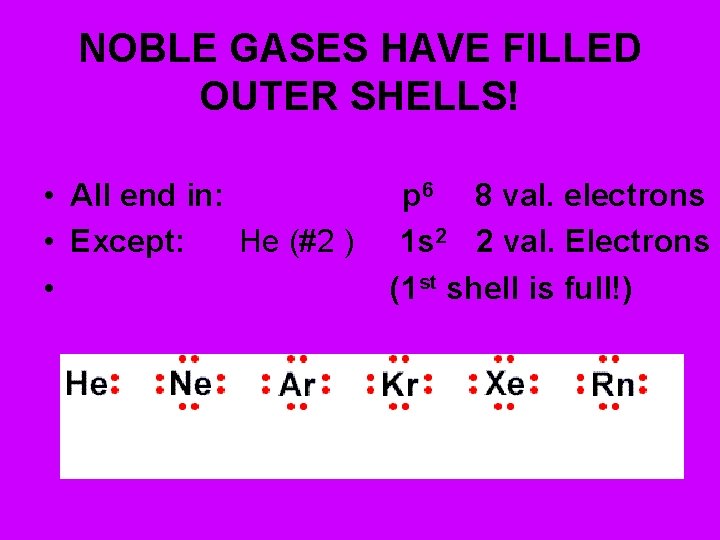
NOBLE GASES – Have filled outer shells • 8 valence electrons - stable octet! • (He – has 2) • Ar (#18) • 1 s 22 p 6 3 s 23 p 6 (8 val. ) • He (#2 ) 1 s 2 (1 st shell – full) • Noble Gases end in “p 6”

NOBLE GASES HAVE FILLED OUTER SHELLS! • All end in: • Except: He (#2 ) • p 6 8 val. electrons 1 s 2 2 val. Electrons (1 st shell is full!)

EXCEPTIONS • Half filled and filled sets of s & d orbitals very stable: • • Expected: Cr Actual: • • Ar ↑_ 4 s • Complex chemistry of transition elements. Ar 4 s 23 d 4 Ar 4 s 13 d 5 ↑_ ↑_ _↑_ 3 d
![EXCEPTIONS cont Cu 29 Predicted configuration Ar 4 s 2 EXCEPTIONS (cont. ) • Cu #29 • Predicted configuration: • [Ar] 4 s 2](https://slidetodoc.com/presentation_image_h2/69e4d608d0278ffa99882b36141544a9/image-26.jpg)
EXCEPTIONS (cont. ) • Cu #29 • Predicted configuration: • [Ar] 4 s 2 3 d 9 • Actual configuration: • [Ar] 4 s 1 3 d 10 • Note: full “d” is stable.

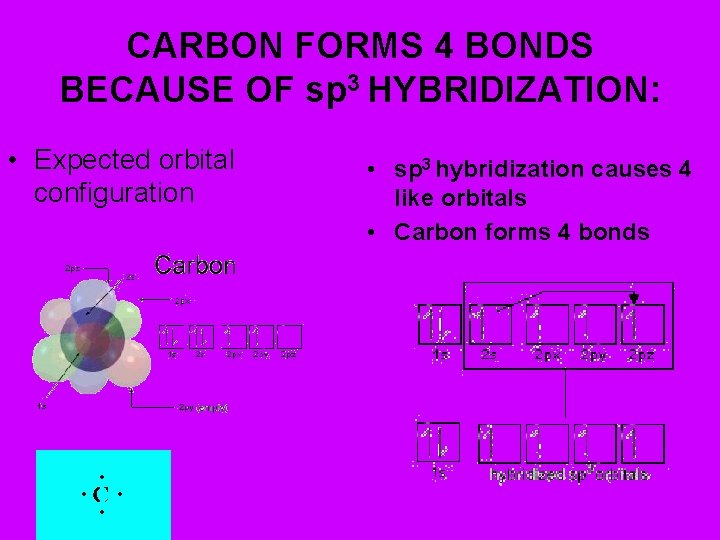
CARBON FORMS 4 BONDS BECAUSE OF sp 3 HYBRIDIZATION: • Expected orbital configuration • sp 3 hybridization causes 4 like orbitals • Carbon forms 4 bonds

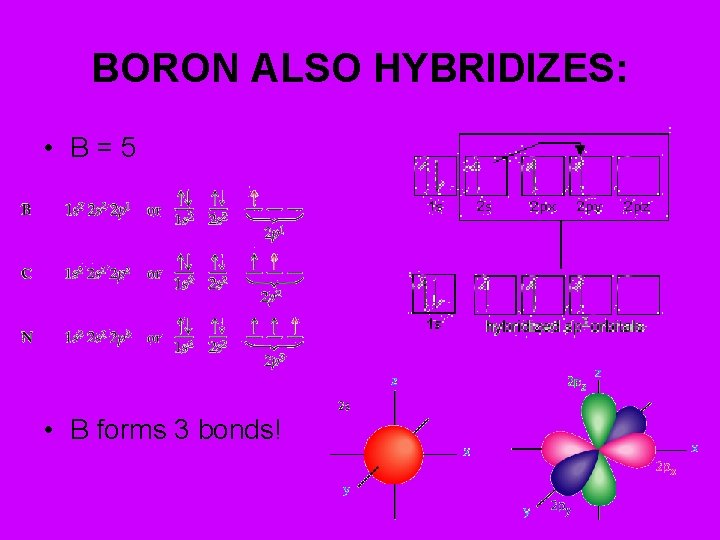
BORON ALSO HYBRIDIZES: • B=5 • B forms 3 bonds!

THE END