5 2 Nuclear Reactions In the nuclear equation
- Slides: 26

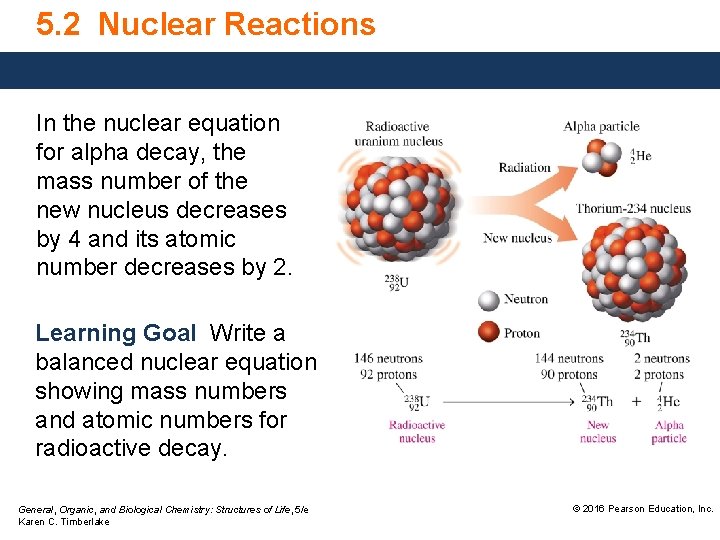
5. 2 Nuclear Reactions In the nuclear equation for alpha decay, the mass number of the new nucleus decreases by 4 and its atomic number decreases by 2. Learning Goal Write a balanced nuclear equation showing mass numbers and atomic numbers for radioactive decay. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

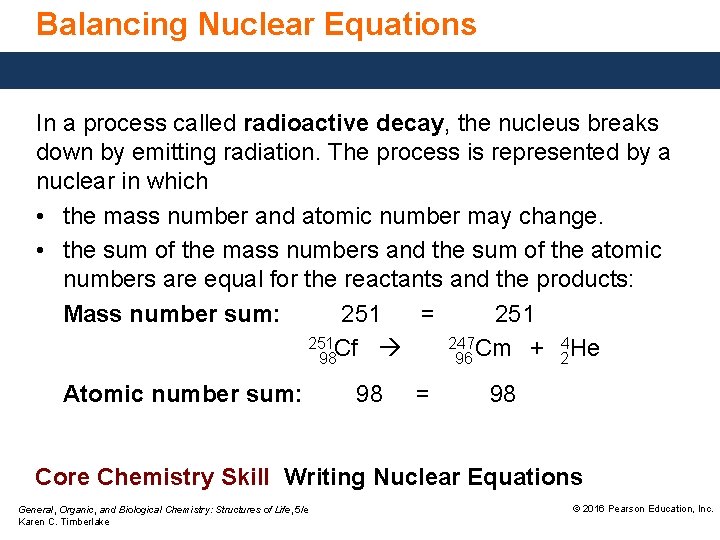
Balancing Nuclear Equations In a process called radioactive decay, the nucleus breaks down by emitting radiation. The process is represented by a nuclear in which • the mass number and atomic number may change. • the sum of the mass numbers and the sum of the atomic numbers are equal for the reactants and the products: Mass number sum: 251 = 251 Cf 247 Cm + 4 He 98 96 2 Atomic number sum: 98 = 98 Core Chemistry Skill Writing Nuclear Equations General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

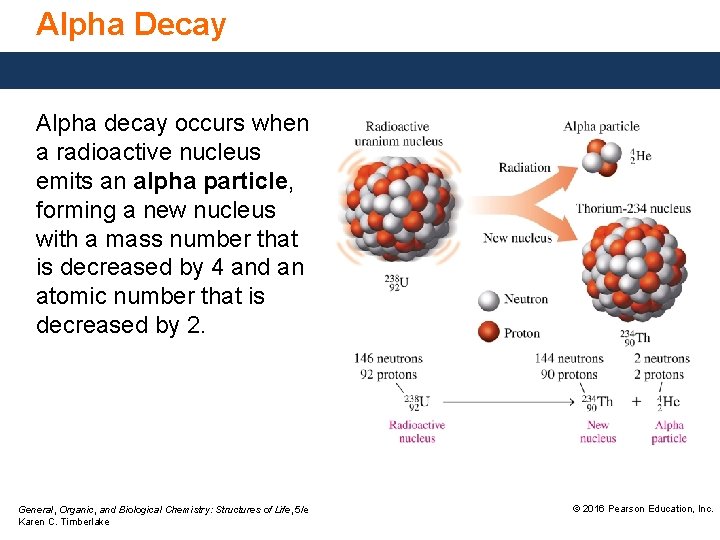
Alpha Decay Alpha decay occurs when a radioactive nucleus emits an alpha particle, forming a new nucleus with a mass number that is decreased by 4 and an atomic number that is decreased by 2. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

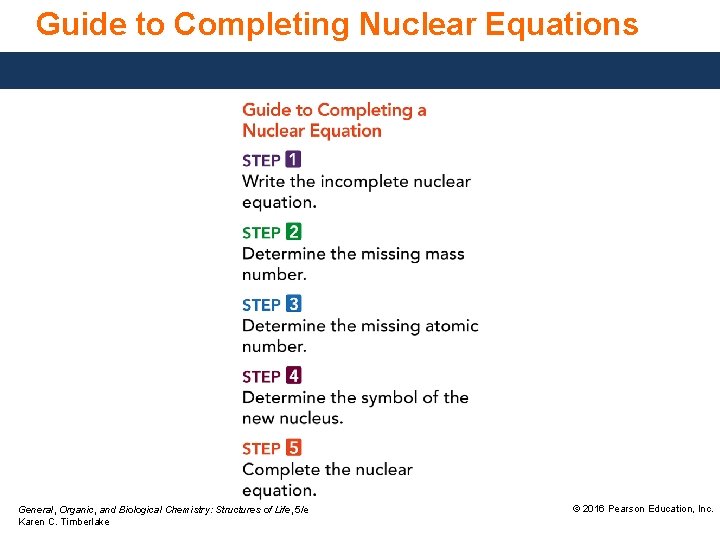
Guide to Completing Nuclear Equations General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

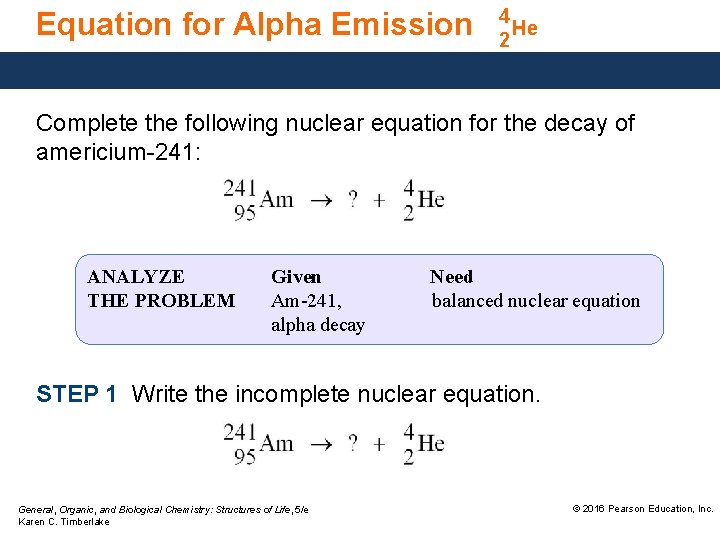
Equation for Alpha Emission 4 He 2 Complete the following nuclear equation for the decay of americium-241: ANALYZE THE PROBLEM Given Am-241, alpha decay Need balanced nuclear equation STEP 1 Write the incomplete nuclear equation. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

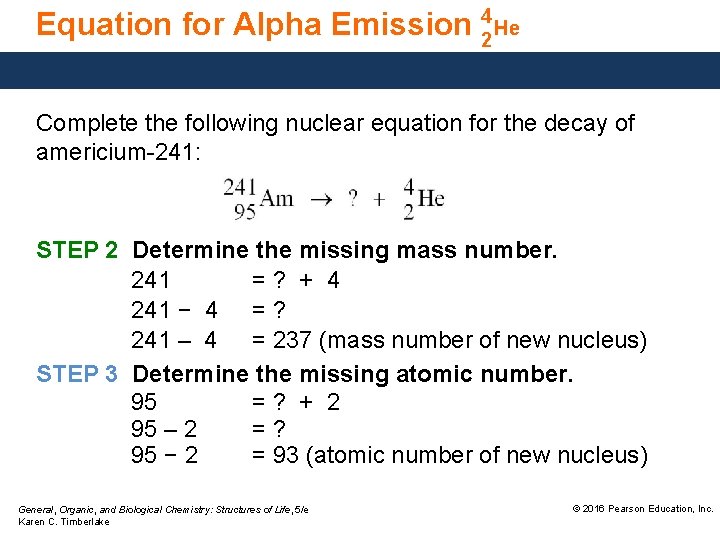
Equation for Alpha Emission 42 He Complete the following nuclear equation for the decay of americium-241: STEP 2 Determine the missing mass number. 241 =? + 4 241 − 4 = ? 241 – 4 = 237 (mass number of new nucleus) STEP 3 Determine the missing atomic number. 95 =? + 2 95 – 2 =? 95 − 2 = 93 (atomic number of new nucleus) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

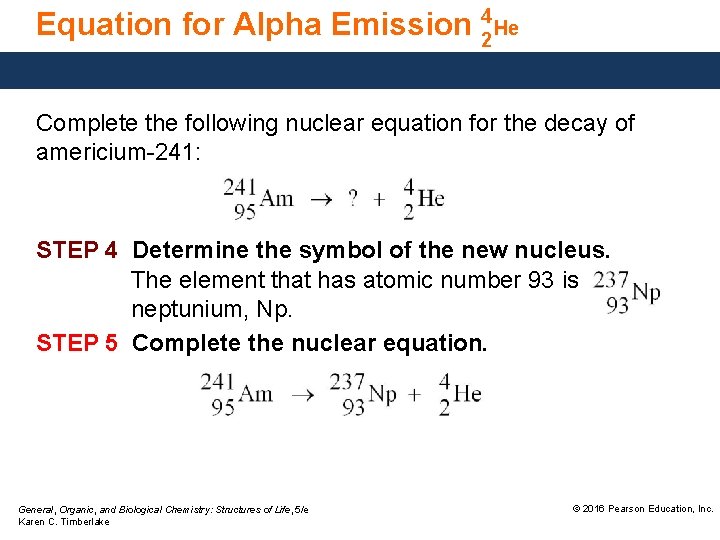
Equation for Alpha Emission 42 He Complete the following nuclear equation for the decay of americium-241: STEP 4 Determine the symbol of the new nucleus. The element that has atomic number 93 is neptunium, Np. STEP 5 Complete the nuclear equation. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

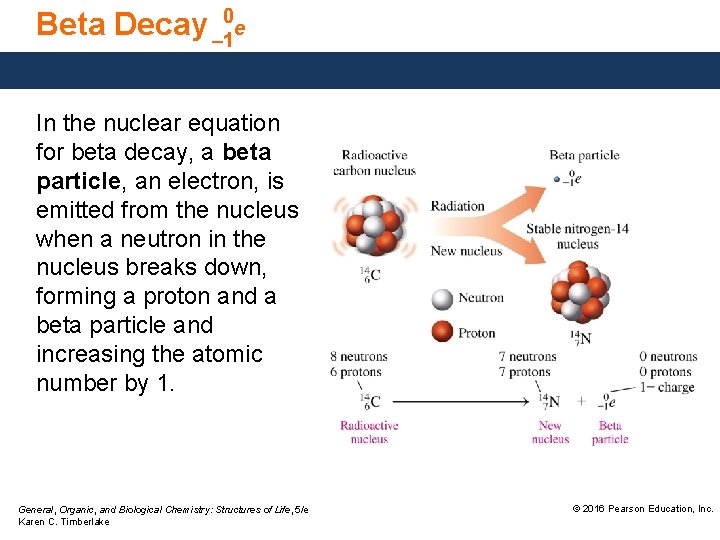
Beta Decay – 10 e In the nuclear equation for beta decay, a beta particle, an electron, is emitted from the nucleus when a neutron in the nucleus breaks down, forming a proton and a beta particle and increasing the atomic number by 1. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

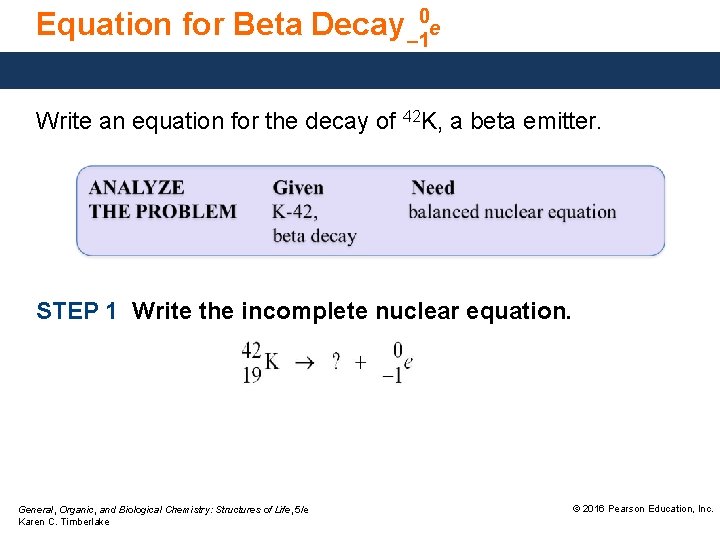
Equation for Beta Decay – 10 e Write an equation for the decay of 42 K, a beta emitter. STEP 1 Write the incomplete nuclear equation. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

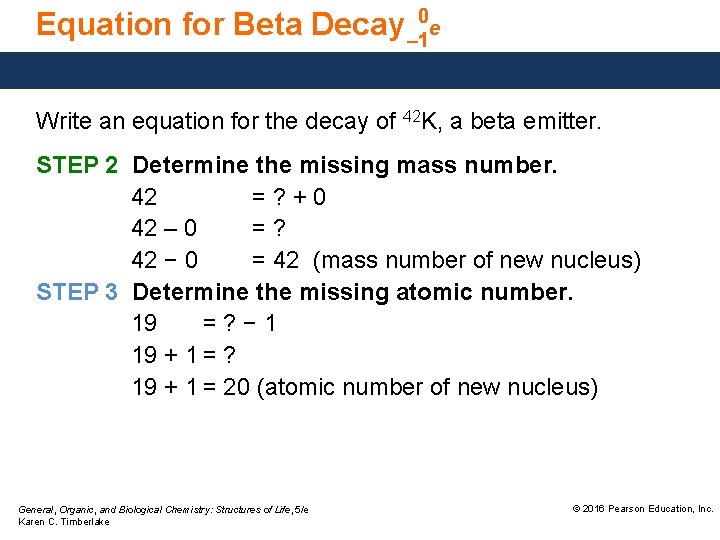
Equation for Beta Decay – 10 e Write an equation for the decay of 42 K, a beta emitter. STEP 2 Determine the missing mass number. 42 =? +0 42 – 0 =? 42 − 0 = 42 (mass number of new nucleus) STEP 3 Determine the missing atomic number. 19 =? − 1 19 + 1 = ? 19 + 1 = 20 (atomic number of new nucleus) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

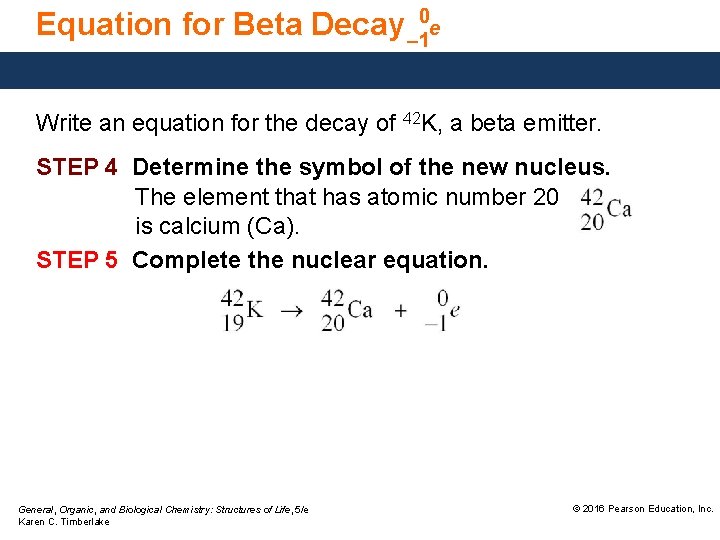
Equation for Beta Decay – 10 e Write an equation for the decay of 42 K, a beta emitter. STEP 4 Determine the symbol of the new nucleus. The element that has atomic number 20 is calcium (Ca). STEP 5 Complete the nuclear equation. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

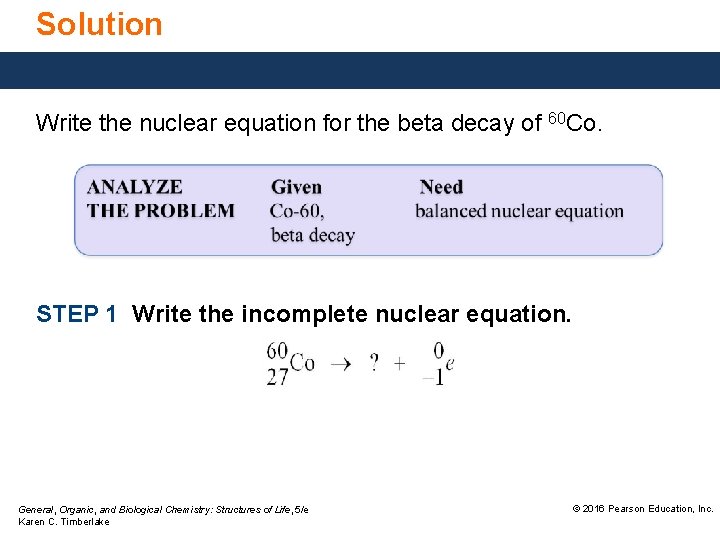
Study Check Write the nuclear equation for the beta decay of 60 Co. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution Write the nuclear equation for the beta decay of 60 Co. STEP 1 Write the incomplete nuclear equation. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

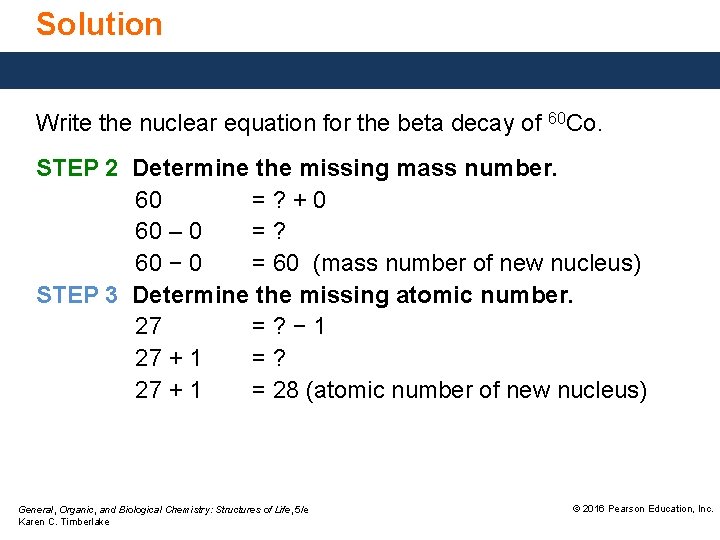
Solution Write the nuclear equation for the beta decay of 60 Co. STEP 2 Determine the missing mass number. 60 =? +0 60 – 0 =? 60 − 0 = 60 (mass number of new nucleus) STEP 3 Determine the missing atomic number. 27 =? − 1 27 + 1 =? 27 + 1 = 28 (atomic number of new nucleus) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

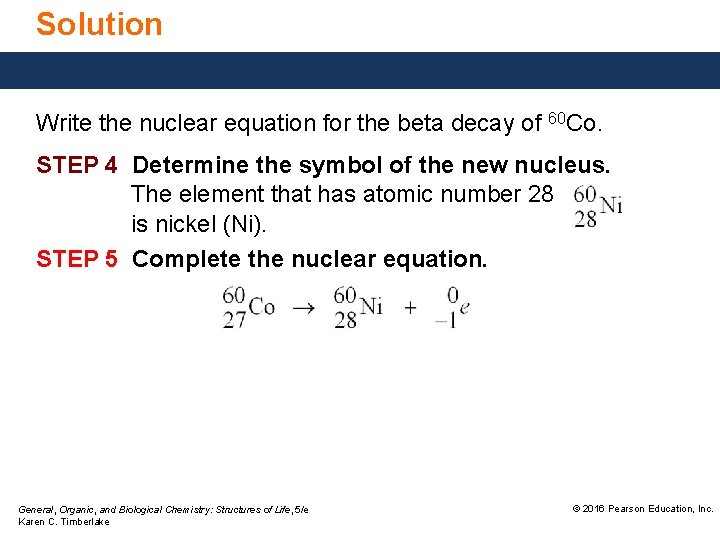
Solution Write the nuclear equation for the beta decay of 60 Co. STEP 4 Determine the symbol of the new nucleus. The element that has atomic number 28 is nickel (Ni). STEP 5 Complete the nuclear equation. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

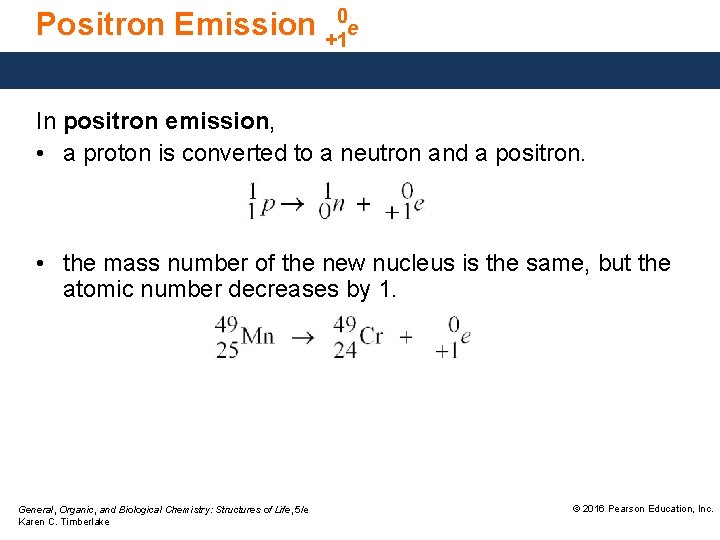
Positron Emission +10 e In positron emission, • a proton is converted to a neutron and a positron. • the mass number of the new nucleus is the same, but the atomic number decreases by 1. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

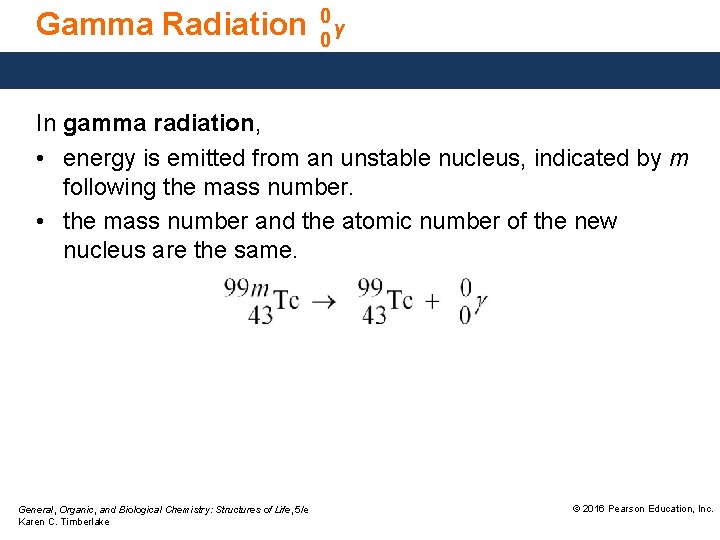
Gamma Radiation 0 γ 0 In gamma radiation, • energy is emitted from an unstable nucleus, indicated by m following the mass number. • the mass number and the atomic number of the new nucleus are the same. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

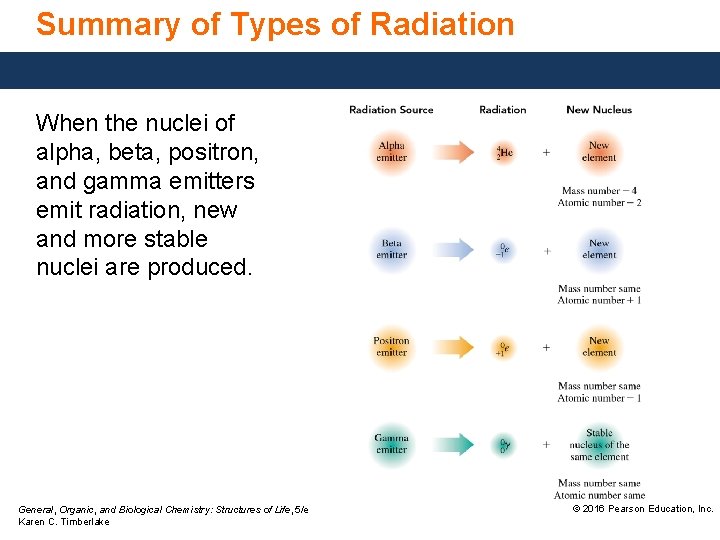
Summary of Types of Radiation When the nuclei of alpha, beta, positron, and gamma emitters emit radiation, new and more stable nuclei are produced. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

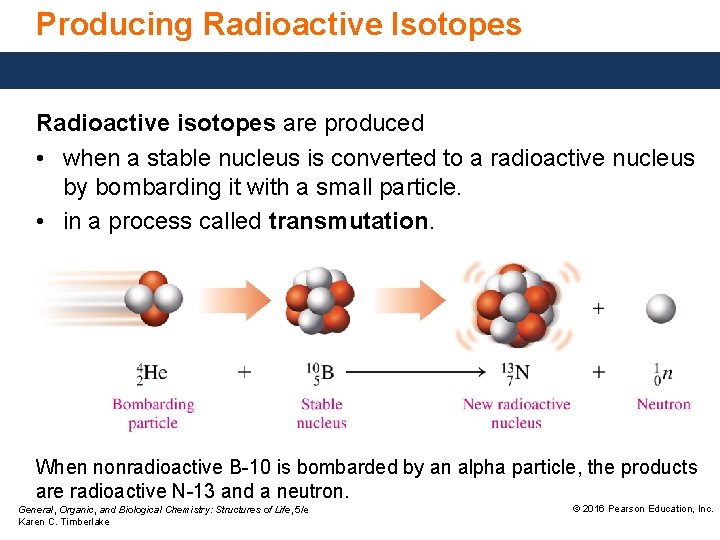
Producing Radioactive Isotopes Radioactive isotopes are produced • when a stable nucleus is converted to a radioactive nucleus by bombarding it with a small particle. • in a process called transmutation. When nonradioactive B-10 is bombarded by an alpha particle, the products are radioactive N-13 and a neutron. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

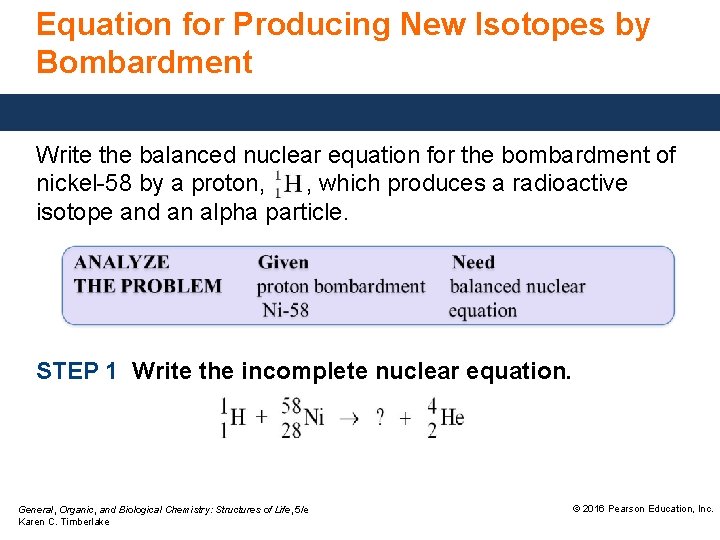
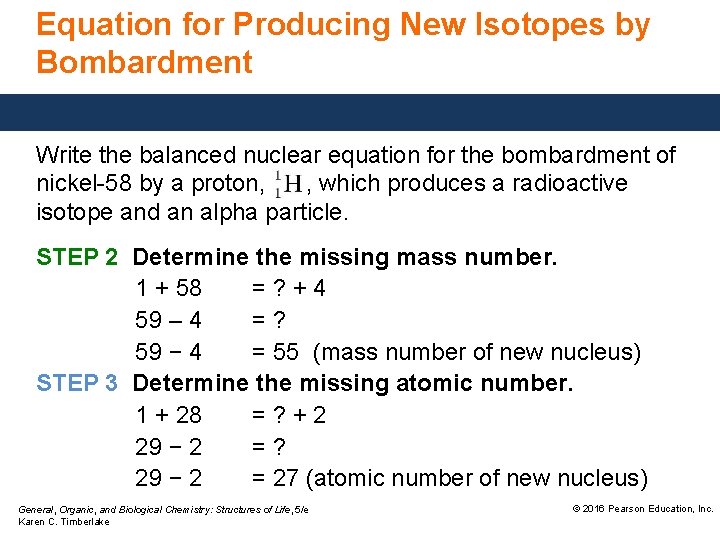
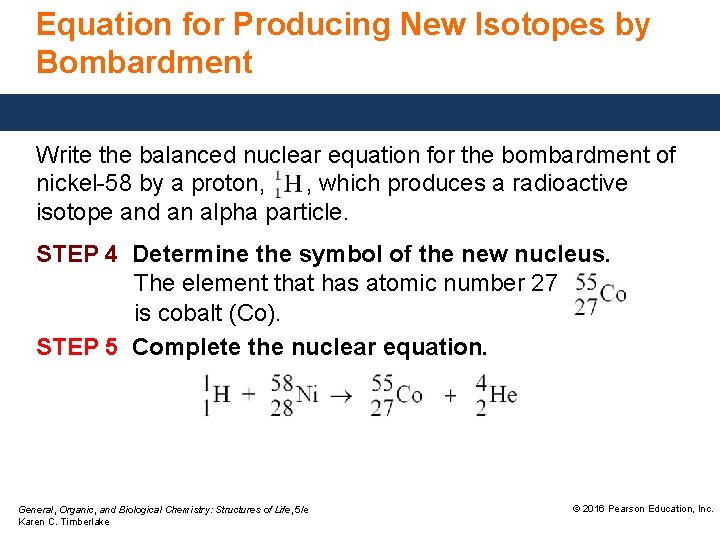
Equation for Producing New Isotopes by Bombardment Write the balanced nuclear equation for the bombardment of nickel-58 by a proton, , which produces a radioactive isotope and an alpha particle. STEP 1 Write the incomplete nuclear equation. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Equation for Producing New Isotopes by Bombardment Write the balanced nuclear equation for the bombardment of nickel-58 by a proton, , which produces a radioactive isotope and an alpha particle. STEP 2 Determine the missing mass number. 1 + 58 =? +4 59 – 4 =? 59 − 4 = 55 (mass number of new nucleus) STEP 3 Determine the missing atomic number. 1 + 28 =? +2 29 − 2 =? 29 − 2 = 27 (atomic number of new nucleus) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Equation for Producing New Isotopes by Bombardment Write the balanced nuclear equation for the bombardment of nickel-58 by a proton, , which produces a radioactive isotope and an alpha particle. STEP 4 Determine the symbol of the new nucleus. The element that has atomic number 27 is cobalt (Co). STEP 5 Complete the nuclear equation. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

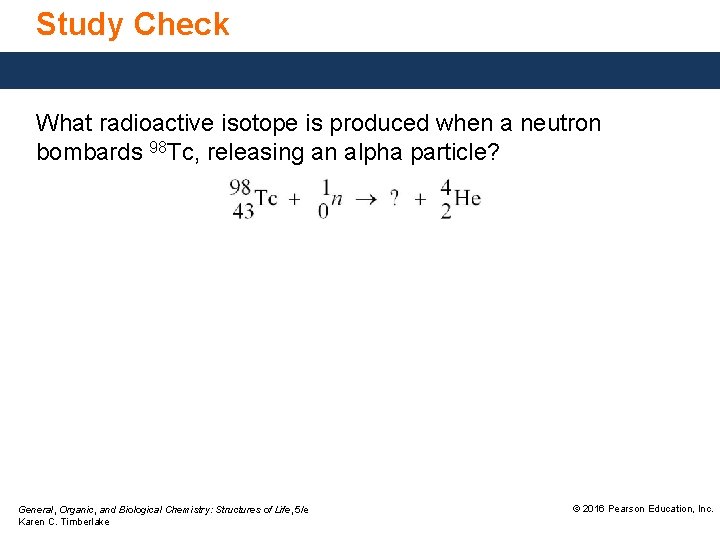
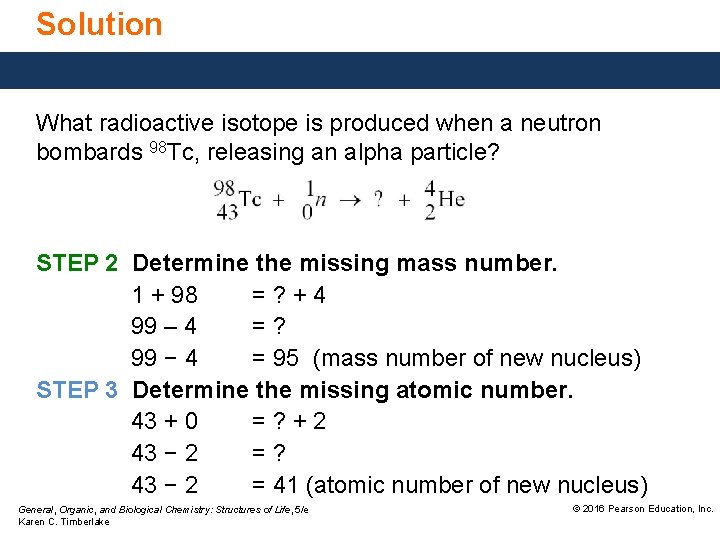
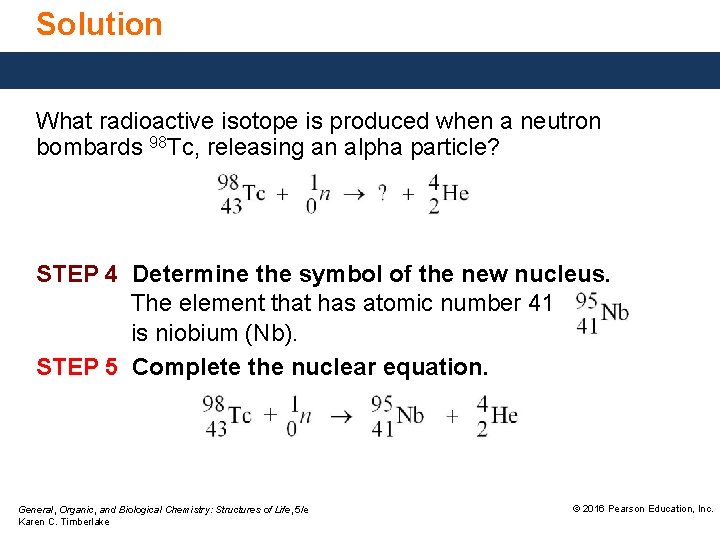
Study Check What radioactive isotope is produced when a neutron bombards 98 Tc, releasing an alpha particle? General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

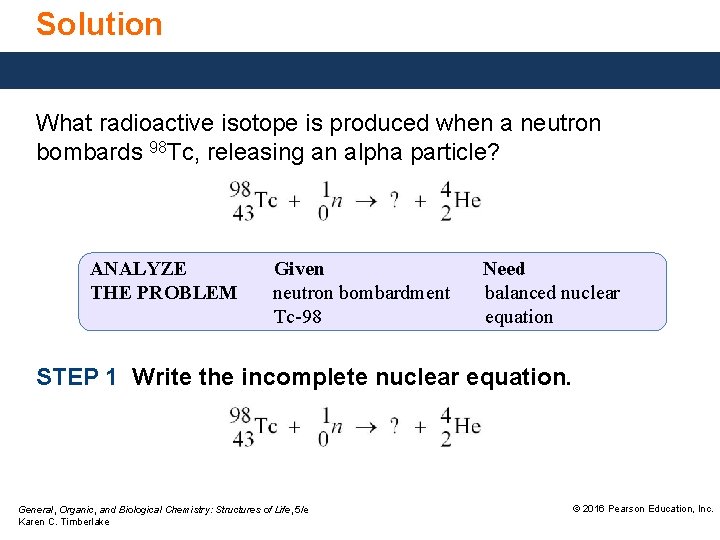
Solution What radioactive isotope is produced when a neutron bombards 98 Tc, releasing an alpha particle? ANALYZE THE PROBLEM Given neutron bombardment Tc-98 Need balanced nuclear equation STEP 1 Write the incomplete nuclear equation. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution What radioactive isotope is produced when a neutron bombards 98 Tc, releasing an alpha particle? STEP 2 Determine the missing mass number. 1 + 98 =? +4 99 – 4 =? 99 − 4 = 95 (mass number of new nucleus) STEP 3 Determine the missing atomic number. 43 + 0 =? +2 43 − 2 =? 43 − 2 = 41 (atomic number of new nucleus) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution What radioactive isotope is produced when a neutron bombards 98 Tc, releasing an alpha particle? STEP 4 Determine the symbol of the new nucleus. The element that has atomic number 41 is niobium (Nb). STEP 5 Complete the nuclear equation. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.