5 2 Composition and Structure of Minerals Objectives
5. 2 Composition and Structure of Minerals Objectives: 1) Identify the characteristics of minerals, 2) Explain how minerals form. 3) List the physical characteristics of minerals that are influenced by their crystalline structure.

What is a Mineral? • Mineral: has 5 characteristics • Occurs naturally • Solid • Definite chemical composition • Atoms are arranged in an orderly pattern • Inorganic (never alive)
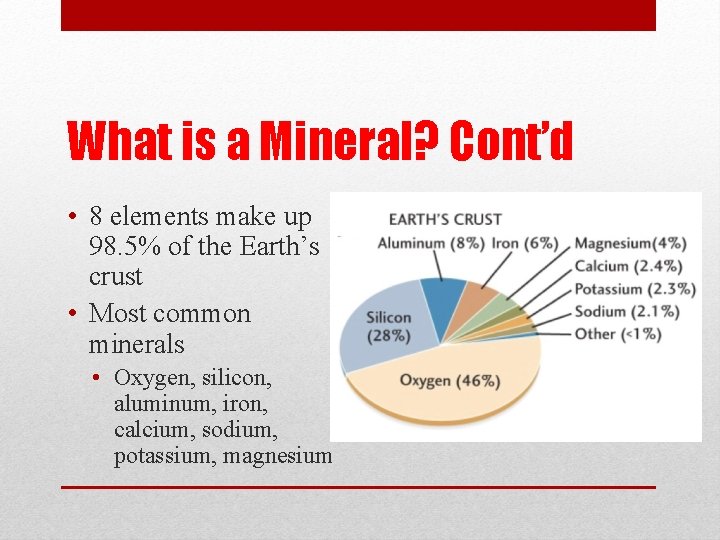
What is a Mineral? Cont’d • 8 elements make up 98. 5% of the Earth’s crust • Most common minerals • Oxygen, silicon, aluminum, iron, calcium, sodium, potassium, magnesium

What is a Mineral? Cont’d • More than 90% of the minerals in Earth’s crust are compounds containing oxygen and silicon • Most minerals are compounds • Native elements: minerals made of single elements • Silver, copper, sulfur, diamond • Rocks: mixtures of minerals
How Minerals Form • 4 ways to create minerals • • Magma Heat Pressure Chemical action • Creating crystals lab

Structure of Minerals • Crystal: a regular geometric solid with smooth surfaces called crystal faces

Crystal Structure • The arrangement of ions, molecules, or atoms in any mineral determines the shape of the crystal • Each type of mineral has its own crystal form
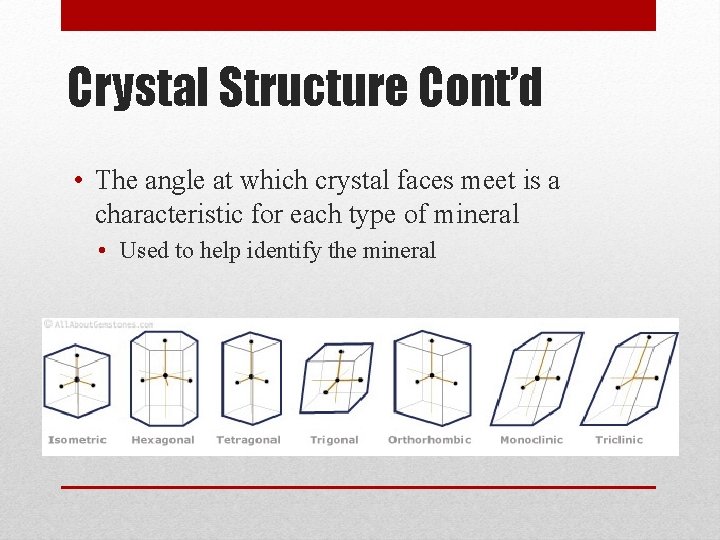
Crystal Structure Cont’d • The angle at which crystal faces meet is a characteristic for each type of mineral • Used to help identify the mineral

Crystal Structure Cont’d • If space is limited when a mineral is forming, there may not be enough room for crystal faces to develop fully, or “grow” • The mineral fills the available space • Still crystalline, but faces are not visible
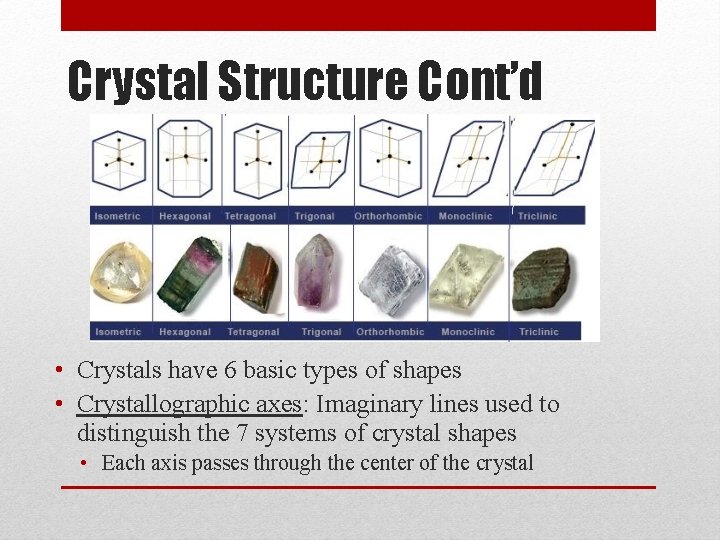
Crystal Structure Cont’d • Crystals have 6 basic types of shapes • Crystallographic axes: Imaginary lines used to distinguish the 7 systems of crystal shapes • Each axis passes through the center of the crystal

Silicates • Silicates: minerals that are compounds including silicon and oxygen • May also contain 1 or more metallic elements • Few do not contain metal • >90% of the minerals in Earth’s crust
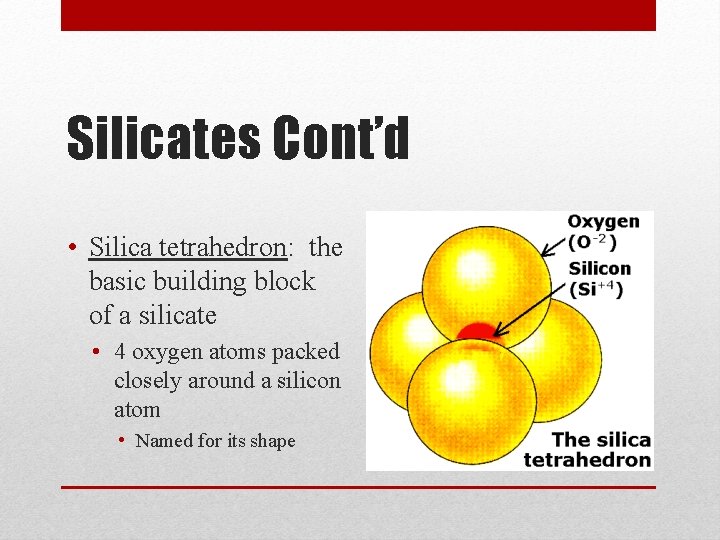
Silicates Cont’d • Silica tetrahedron: the basic building block of a silicate • 4 oxygen atoms packed closely around a silicon atom • Named for its shape
Crystal Structure and Physical Properties • Minerals are solid b/c of crystalline structures • Atoms, ion, and molecules are closely packed by strong chemical bonds

Crystal Structure and Physical Properties Cont’d • High temperatures breaks the bonds and causes minerals to melt then to vaporize • The temperatures at which a mineral melts and vaporizes are characteristic of the mineral • Can sometimes identify a mineral

Crystal Structure and Physical Properties Cont’d • Cleavage: tendency to split along definite planes • Splits along weak bonds between atoms, ions, or molecules of the mineral

Crystal Structure and Physical Properties Cont’d • Hardness of a mineral depends on the arrangement of its ions, atoms, or molecules and on the strength of the chemical bonds between them
Crystal Structure and Physical Properties Cont’d • Density depends on the masses of the atoms in the mineral but also on how they are arranged

5. 2 Exit Ticket • Answer the following questions. • Use complete sentences. • You may NOT use your book. You MAY use your notes. • This is a quiz grade. 1) Identify the characteristics of minerals, 2) Explain how minerals form. 3) List the physical characteristics of minerals that are influenced by their crystalline structure.
- Slides: 18