5 2 Atomic Structure Theory Review of Atom






- Slides: 16

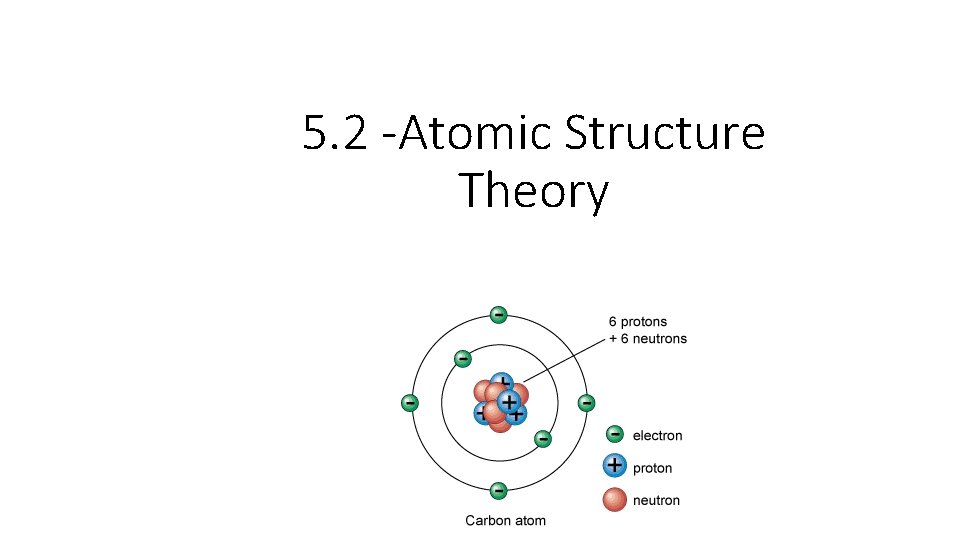
5. 2 -Atomic Structure Theory

Review of Atom History Timeline! • Democritus – theorized that everything was made of small particles 460 – 370 BC • Dalton – 1803 - All matter is made up atoms – Billiard Ball Model • Becquerel – 1896 - discorvered radioactivity and X-ray • Crooke – 1859 -1870 - discovered that the atom had charged particles • Chadwick – 1932 - discovered the neutron • JJ Thompson – 1897 - discovered the electron • R. A. Milllikan – 1903 -1904 - found the electron and its mass • Shrodinger -1926 - developed equations to predict the location of a electron • Rutherford -1911 - discovered the nucleus with the Gold Foil Experiment

• Marie Curie - 1898 - discovered Polonium and Radium – Did extensive research with radioactivity • Niels Bohr - 1913 - create atomic model with electron energy levels

The Atom • Atoms – the smallest part of an element that cannot be broken down by chemical reaction. - The basic unit of all matter

Actual Mass The Atomic Mass Unit (amu) is a standardized unit to measure atomic mass. Carbon -12 is used as the standard. 1/12 th of a Carbon-12 atom is 1 amu. • Proton – 1. 007276 amu • Neutron – 1. 008665 amu • Electron -. 0005486 amu

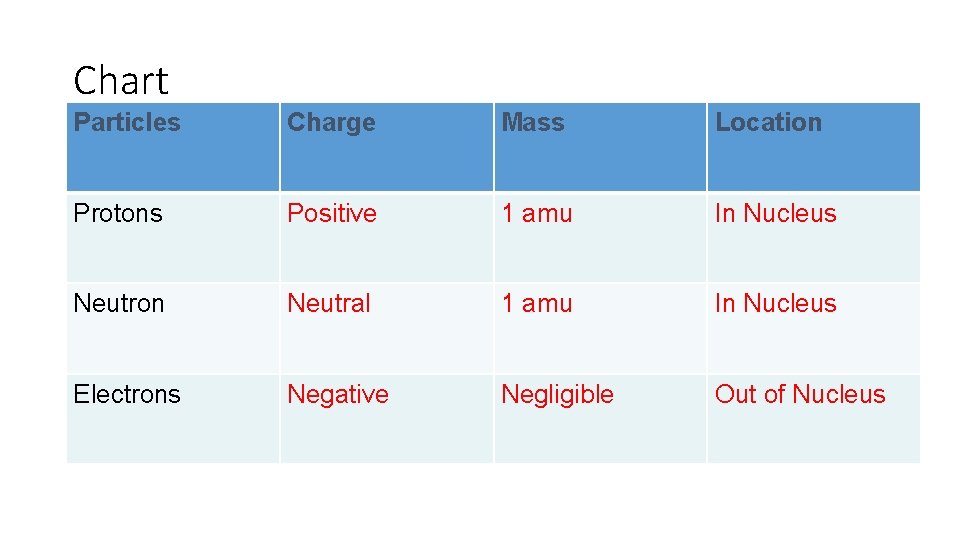
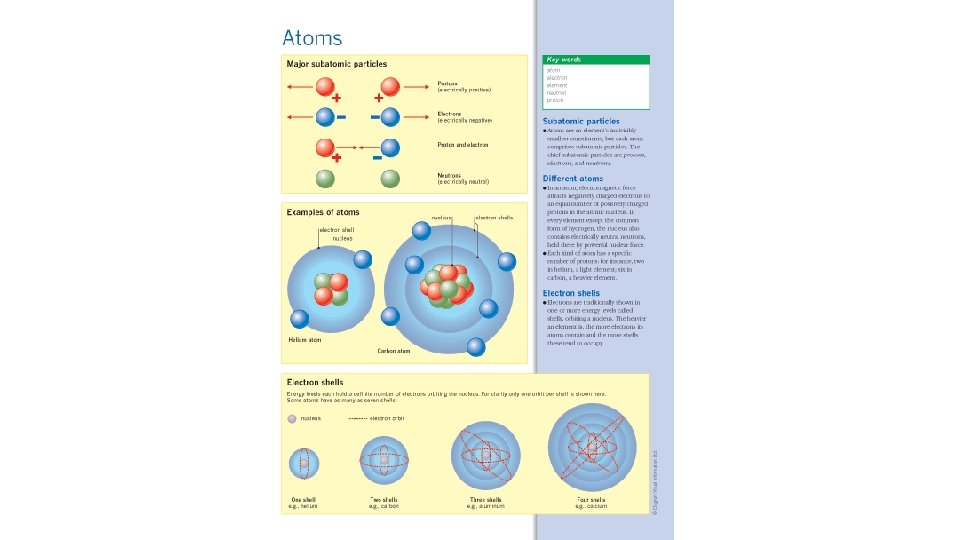
Subatomic Particles Neutron (n 0) – neutrally charged particle making up the nucleus of the atom. Mass = 1 atomic mass unit (amu) • Proton (p+) – positively charged particles making up the nucleus of the atom. Mass = 1 atomic mass unit (amu) • Electron (e-) – Negatively charged particle found in energy levels outside the nucleus. Mass is negligible – not significant

Chart Particles Charge Mass Location Protons Positive 1 amu In Nucleus Neutron Neutral 1 amu In Nucleus Electrons Negative Negligible Out of Nucleus


• Atomic Number - number of protons in an atom. • All atoms of the same element have the same atomic number • All neutral atoms have equal protons and electrons • The number of neutrons = mass number – atomic number

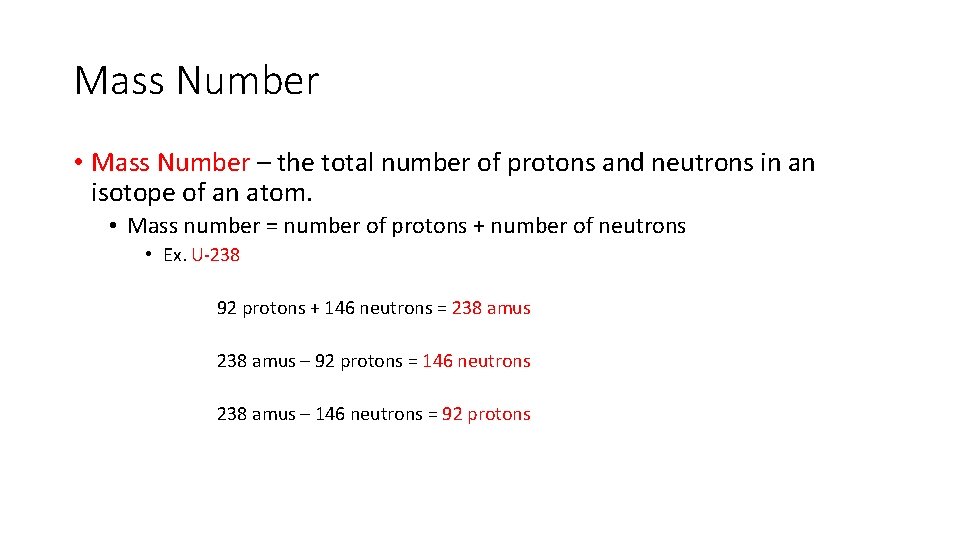
Mass Number • Mass Number – the total number of protons and neutrons in an isotope of an atom. • Mass number = number of protons + number of neutrons • Ex. U-238 92 protons + 146 neutrons = 238 amus – 92 protons = 146 neutrons 238 amus – 146 neutrons = 92 protons

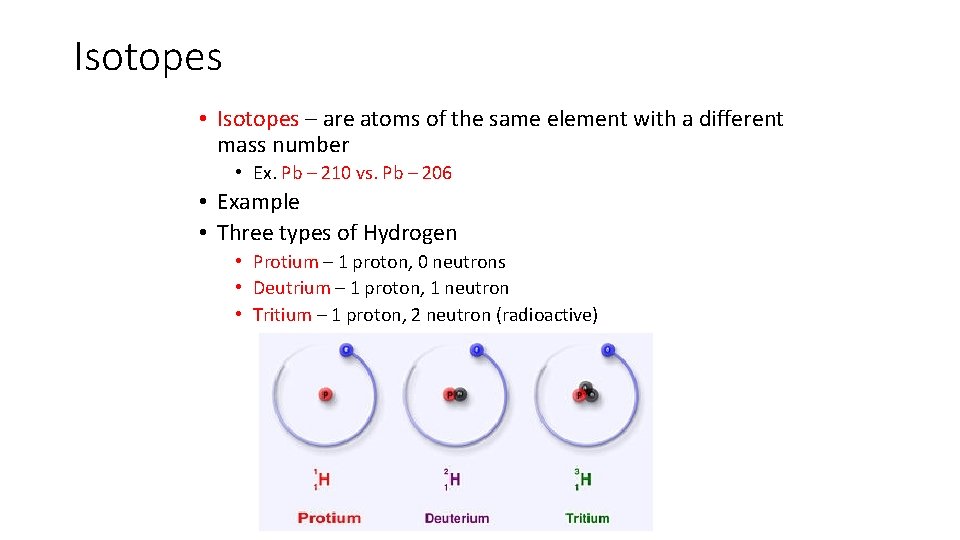
Isotopes • Isotopes – are atoms of the same element with a different mass number • Ex. Pb – 210 vs. Pb – 206 • Example • Three types of Hydrogen • Protium – 1 proton, 0 neutrons • Deutrium – 1 proton, 1 neutron • Tritium – 1 proton, 2 neutron (radioactive)

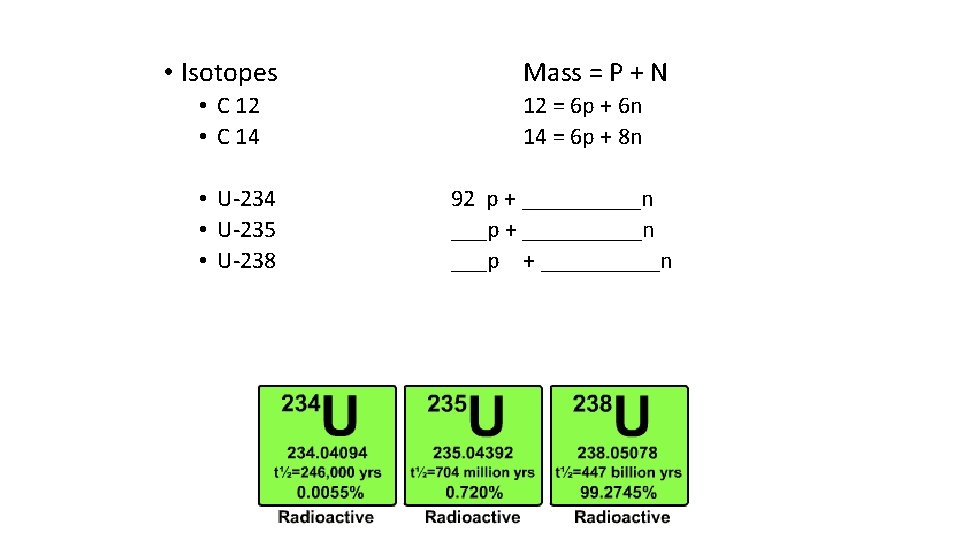
• Isotopes • C 12 • C 14 • U-235 • U-238 Mass = P + N 12 = 6 p + 6 n 14 = 6 p + 8 n 92 p + __________n ___p + _____n

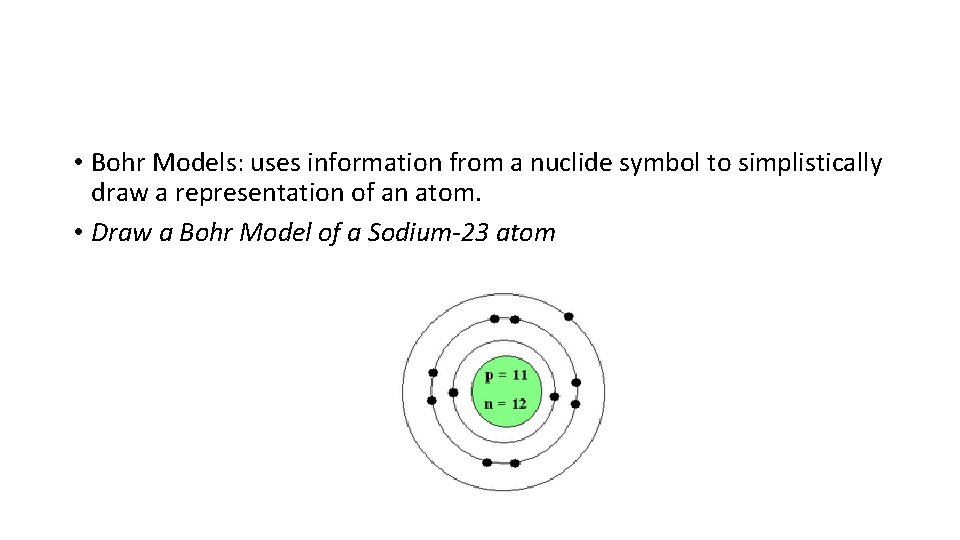
• Bohr Models: uses information from a nuclide symbol to simplistically draw a representation of an atom. • Draw a Bohr Model of a Sodium-23 atom

Atomic Mass • Atomic Mass – the atomic mass of an element is the average mass of the isotopes of that element in a natural sample. Ex. Carbon = 12. 011 amu • Boron 11 – 80. 0% • Boron 10 – 20. 0% • Calculate Atomic Mass:

• Atomic Mass of Boron – 10. 8 amu

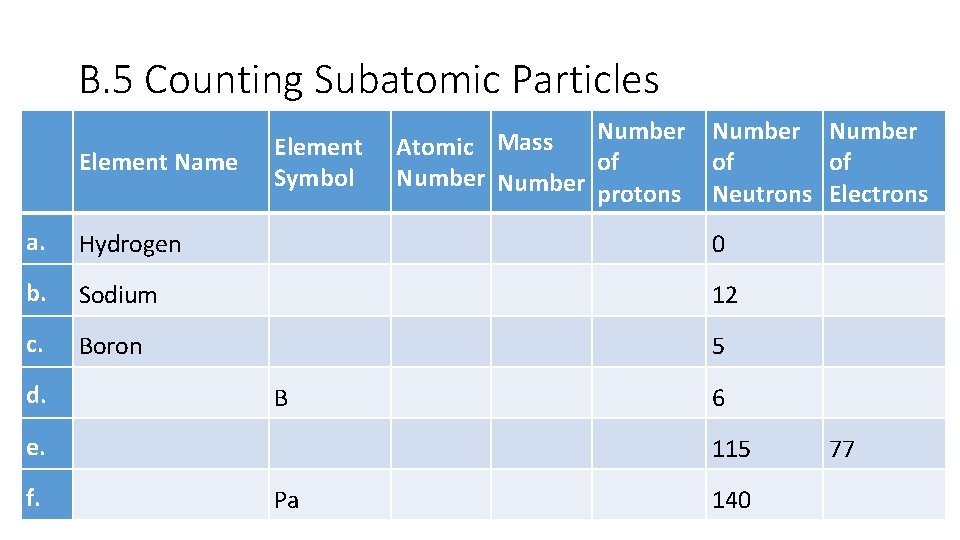
B. 5 Counting Subatomic Particles Element Name Element Symbol Number Mass Atomic of Number protons Number of of Neutrons Electrons a. Hydrogen 0 b. Sodium 12 c. Boron 5 d. B e. f. 6 115 Pa 140 77