5 1 The Combustion of Hydrocarbons Background Organic

5. 1 The Combustion of Hydrocarbons
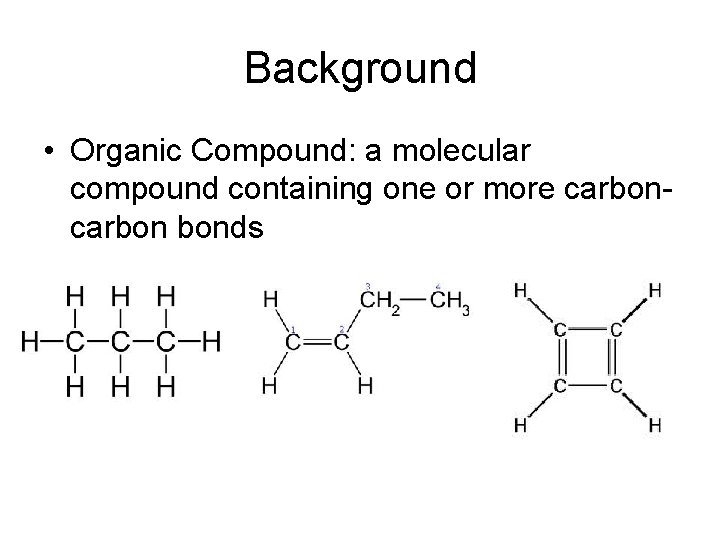
Background • Organic Compound: a molecular compound containing one or more carbon bonds

• Many fuels contain impurities such as sulfur compounds and heavy metals. • These impurities cause air pollution • The burning of hydrocarbons is a large contributing factor to the release of carbon dioxide into the atmosphere. • Carbon dioxide is one green house gas that contributes to theory* of global warming. *reminder that scientific theory =/= everyday theory

Combustion Reaction • Requires the presence of oxygen for complete combustion • The reactants are any material and oxygen • The general equation for a complete combustion reaction of a hydrocarbon is: Hydrocarbon + oxygen → carbon dioxide + water C 3 H 8 (g) + O 2 (g) → CO 2 (g) + H 2 O (g) C 3 H 8 (g) + 5 O 2 (g) → 3 CO 2 (g) + 4 H 2 O (g)
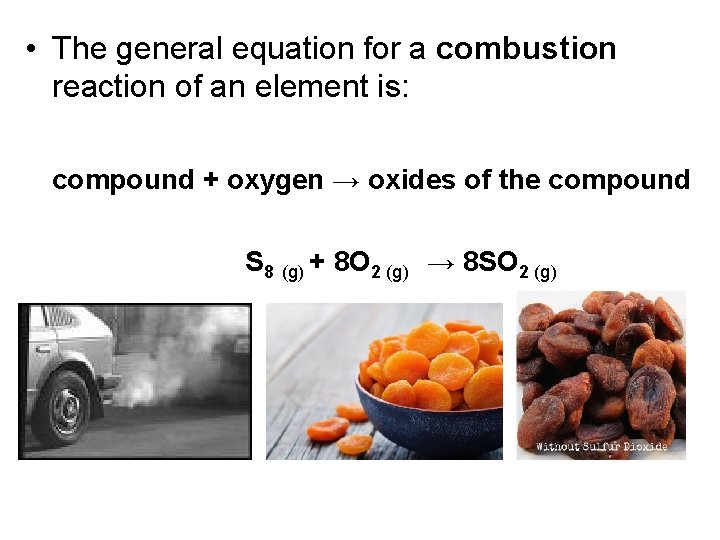
• The general equation for a combustion reaction of an element is: compound + oxygen → oxides of the compound S 8 (g) + 8 O 2 (g) → 8 SO 2 (g)

Incomplete Combustion • Combustion of a hydrocarbon occurs when the supply of oxygen is limited. • It is said to be “fuel rich” • Flames from incomplete combustion are often yellow, sooty and considerably cooler than flames from complete combustion. • They produce a wider range of products and are generally represented by more than one chemical equation
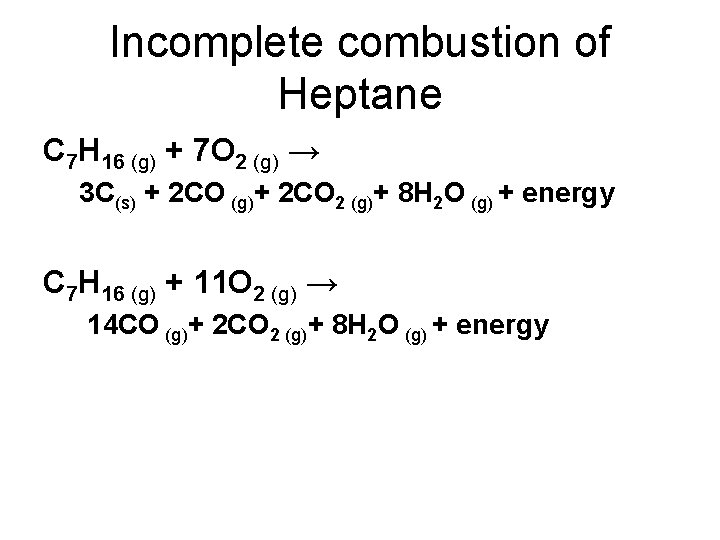
Incomplete combustion of Heptane C 7 H 16 (g) + 7 O 2 (g) → 3 C(s) + 2 CO (g)+ 2 CO 2 (g)+ 8 H 2 O (g) + energy C 7 H 16 (g) + 11 O 2 (g) → 14 CO (g)+ 2 CO 2 (g)+ 8 H 2 O (g) + energy

Fire Fighting • We can stop combustion reactions in several ways. • Fire requires oxygen, heat and a fuel source to continue to burn. Attacking any of these factors can reduce or stop a fire. • Water: Water serves to cool down a fire, absorbing some of the heat from a combustion reaction. • Smothering: Using a blanket overtop or otherwise reducing the oxygen intake, will limit the fire’s ability to burn (complete->incomplete->no combustion)

Fire Extinguishers: There are different kinds of fire extinguishers developed for the variety of fires that exist (combustible liquid, electrical, etc) 3 general types: - Water: They propel water to cool the fire. - Dry Chemical: These serve to cut off the fuel source from oxygen by covering it in a fine powder. - CO 2: These propel liquid CO 2 that expands into a snowy foam. It smothers the fire and absorbs heat from the fire as it evaporates into a gas.

Using page 194 -195 address the concerns related to incomplete combustion
- Slides: 10