5 1 Synthesis and Decomposition Reactions Evidence of





- Slides: 16

5. 1 Synthesis and Decomposition Reactions

Evidence of Chemical Change • Chemical reactions involve chemical changes • Chemical reactions involve the production of new materials (i. e. when wood burns in a campfire, or photosynthesis where water and CO 2 are changed to sugar) • It is not always easy to tell if a chemical change has happened 5. 1

Evidence of Chemical Change • 6 clues a chemical change has happened: (1) A precipitate is formed; a new product that is an insoluble solid (2) A gas is formed (3) A change of color is observed 5. 1

Evidence of Chemical Change • 6 clues a chemical change has happened: (4) A new odor might be produced (5) A change in temperature may occur (6) Light might be produced 5. 1

Classifying Chemical Reactions • The new substances produced during a chemical reaction will depend on the type of the reaction • Understanding the different types of reactions will allow you to identify the products formed • We will study 4 different type of reactions: (1) Synthesis (2) Decomposition (3) Single displacement (4) Double Displacement 5. 1

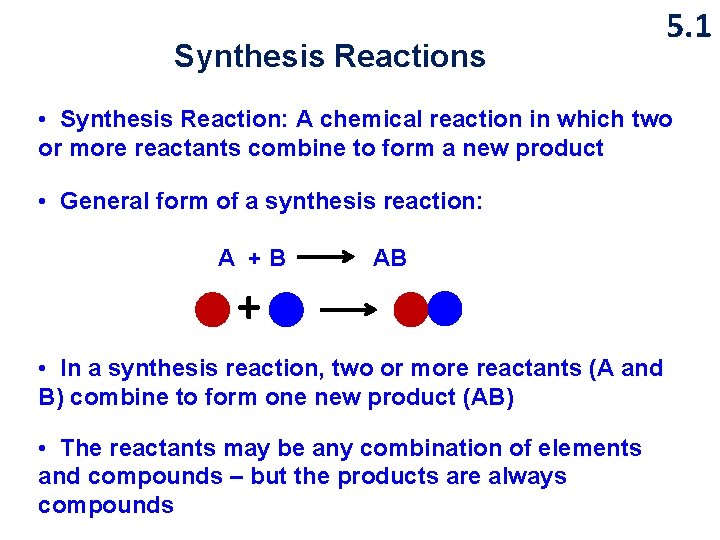
Synthesis Reactions 5. 1 • Synthesis Reaction: A chemical reaction in which two or more reactants combine to form a new product • General form of a synthesis reaction: A +B AB + • In a synthesis reaction, two or more reactants (A and B) combine to form one new product (AB) • The reactants may be any combination of elements and compounds – but the products are always compounds

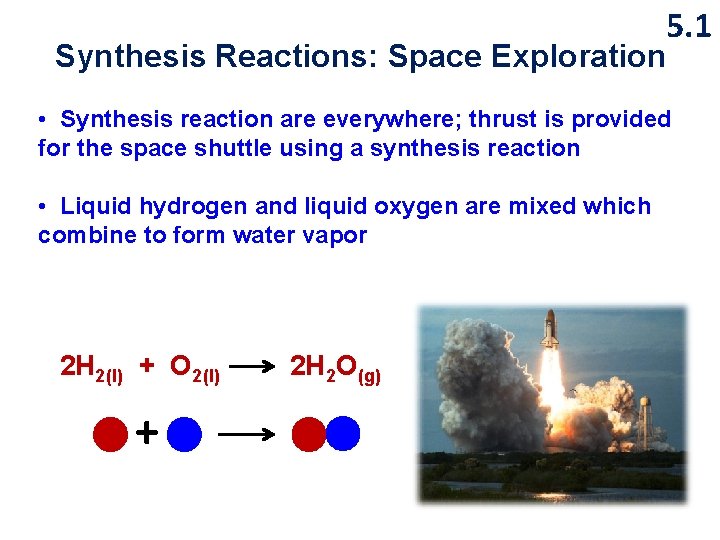
5. 1 Synthesis Reactions: Space Exploration • Synthesis reaction are everywhere; thrust is provided for the space shuttle using a synthesis reaction • Liquid hydrogen and liquid oxygen are mixed which combine to form water vapor 2 H 2(l) + O 2(l) + 2 H 2 O(g)

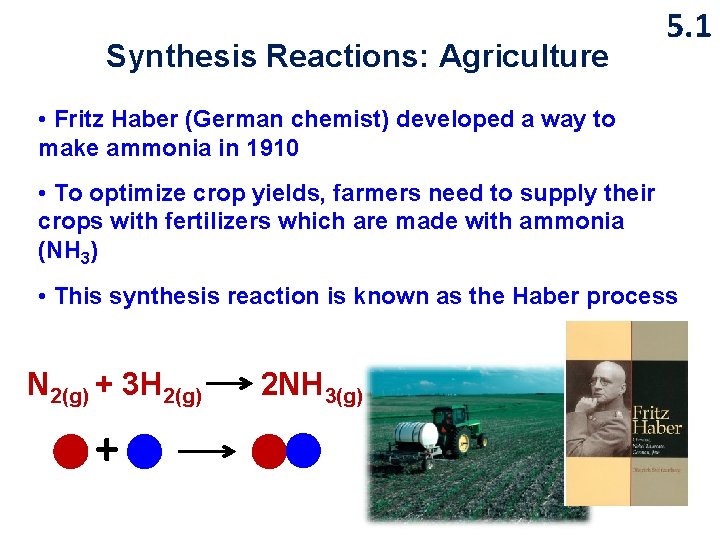
Synthesis Reactions: Agriculture 5. 1 • Fritz Haber (German chemist) developed a way to make ammonia in 1910 • To optimize crop yields, farmers need to supply their crops with fertilizers which are made with ammonia (NH 3) • This synthesis reaction is known as the Haber process N 2(g) + 3 H 2(g) + 2 NH 3(g)

Synthesis Reactions: The Environment 5. 1 • Many atmospheric pollutants are made from synthesis reactions • Nitrogen in the air can combine with oxygen to make nitrogen oxides • Smog is formed in two steps: (1) N 2(g) + O 2(g) 2 NO(g) (colorless gas) (2) 2 NO(g) + O 2(g) 2 NO 2(g) (brown gas) Read STSE case study page 182 – do questions #1 & 2

Synthesizing Binary Ionic Compounds • Knowing how elements combine, it is possible to predict the products of simple synthesis reactions • Example: Complete and Balance the following synthesis reaction: Na(s) + Cl 2(g) 5. 1

Synthesizing Binary Ionic Compounds • Example: Complete and Balance the following synthesis reaction: Na(s) + Cl 2(g) Solution: • Charges: Na 1+ and Cl 1 - (they combine 1: 1 ratio) • Skeleton equation: Na(s) + Cl 2(g) Na. Cl(s) • Balanced equation: 2 Na(s) + Cl 2(g) 2 Na. Cl(s) Do practice questions # 1 -2 on page 184 Do Learning Check questions # 1 -4 on page 185 5. 1

Decomposition Reactions • Decomposition Reaction: A chemical reaction in which a compound breaks down into two or more products • General form of a decomposition reaction: AB A + B + • The products may be any combination of elements and compounds – but the reactants are compounds 5. 1

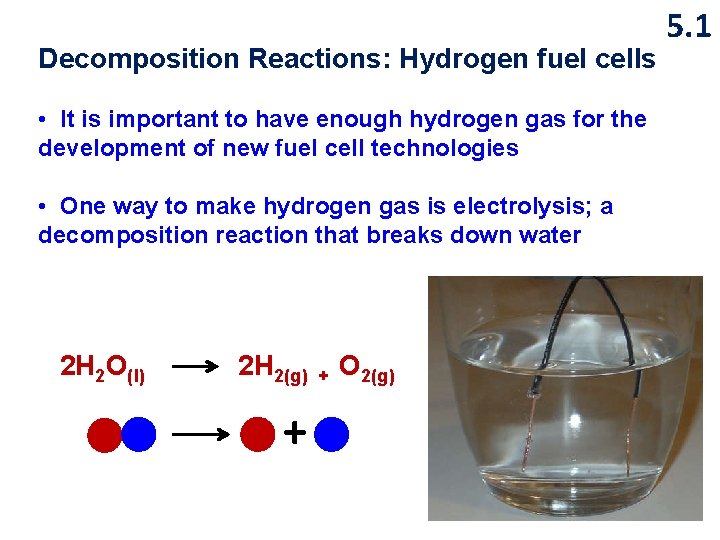
Decomposition Reactions: Hydrogen fuel cells • It is important to have enough hydrogen gas for the development of new fuel cell technologies • One way to make hydrogen gas is electrolysis; a decomposition reaction that breaks down water 2 H 2 O(l) 2 H 2(g) + + O 2(g) 5. 1

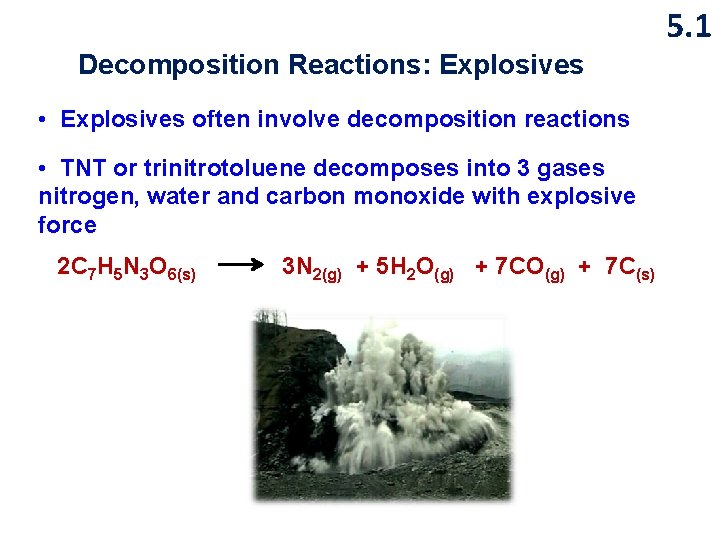
5. 1 Decomposition Reactions: Explosives • Explosives often involve decomposition reactions • TNT or trinitrotoluene decomposes into 3 gases nitrogen, water and carbon monoxide with explosive force 2 C 7 H 5 N 3 O 6(s) 3 N 2(g) + 5 H 2 O(g) + 7 C(s)

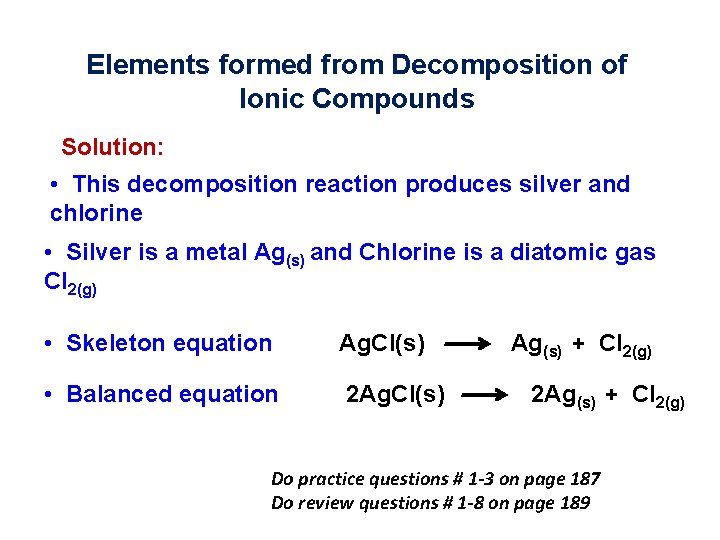
5. 1 Elements formed from Decomposition of Ionic Compounds • Complete and balance the following decomposition reaction: Ag. Cl(s)

Elements formed from Decomposition of Ionic Compounds Solution: • This decomposition reaction produces silver and chlorine • Silver is a metal Ag(s) and Chlorine is a diatomic gas Cl 2(g) • Skeleton equation Ag. Cl(s) • Balanced equation 2 Ag. Cl(s) Ag(s) + Cl 2(g) 2 Ag(s) + Cl 2(g) Do practice questions # 1 -3 on page 187 Do review questions # 1 -8 on page 189