5 1 ENTHALPY OF FORMATION IB CHEMISTRY LESSON

5. 1 ENTHALPY OF FORMATION IB CHEMISTRY

LESSON OBJECTIVES Define standard enthalpy change of formation Write formation equations. Calculate standard enthalpy change of formation
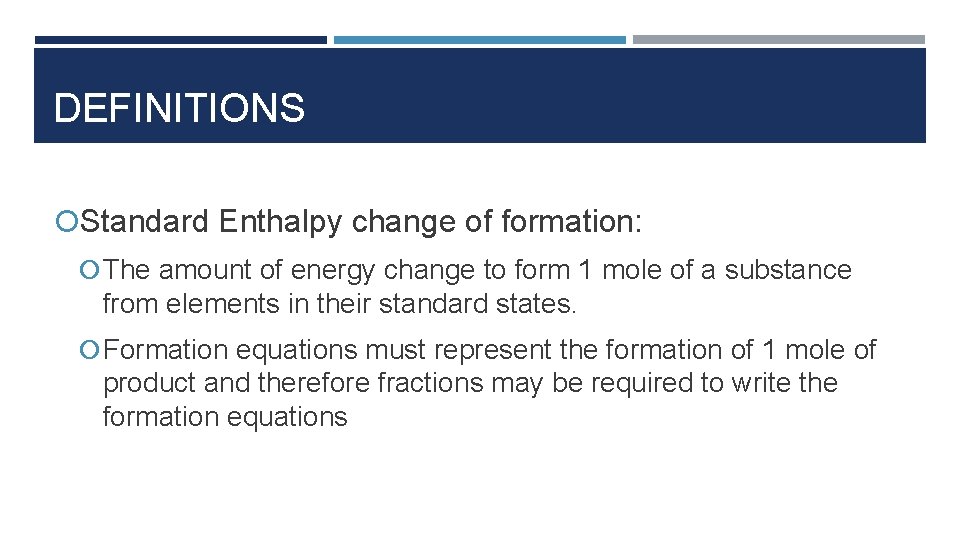
DEFINITIONS Standard Enthalpy change of formation: The amount of energy change to form 1 mole of a substance from elements in their standard states. Formation equations must represent the formation of 1 mole of product and therefore fractions may be required to write the formation equations
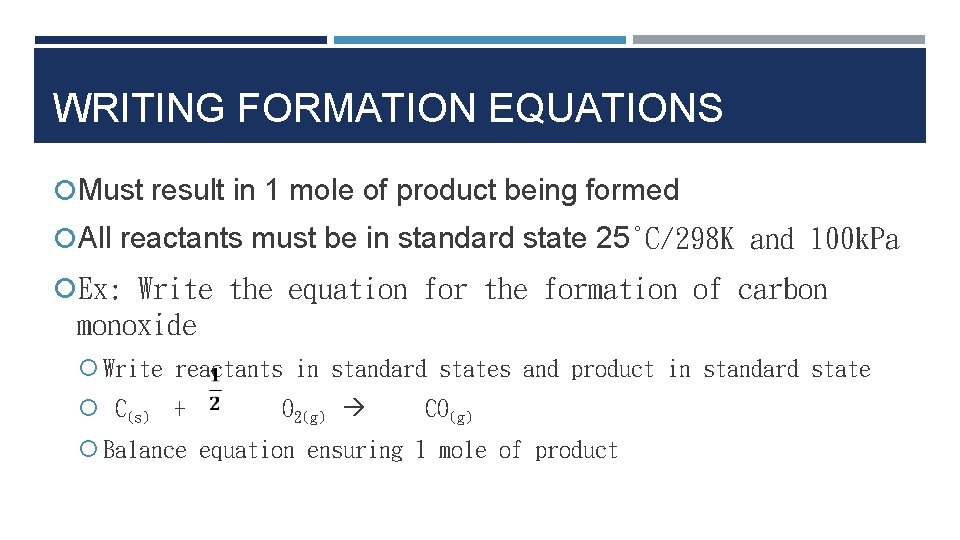
WRITING FORMATION EQUATIONS Must result in 1 mole of product being formed All reactants must be in standard state 25°C/298 K and 100 k. Pa Ex: Write the equation for the formation of carbon monoxide Write reactants in standard states and product in standard state C(s) + O 2(g) CO(g) Balance equation ensuring 1 mole of product
WRITING FORMATION EQUATIONS Write the formation equations for: Silver (I) bromide Ammonia (nitrogen trihydride) CH 3 OH Propane (C 3 H 8) Glucose
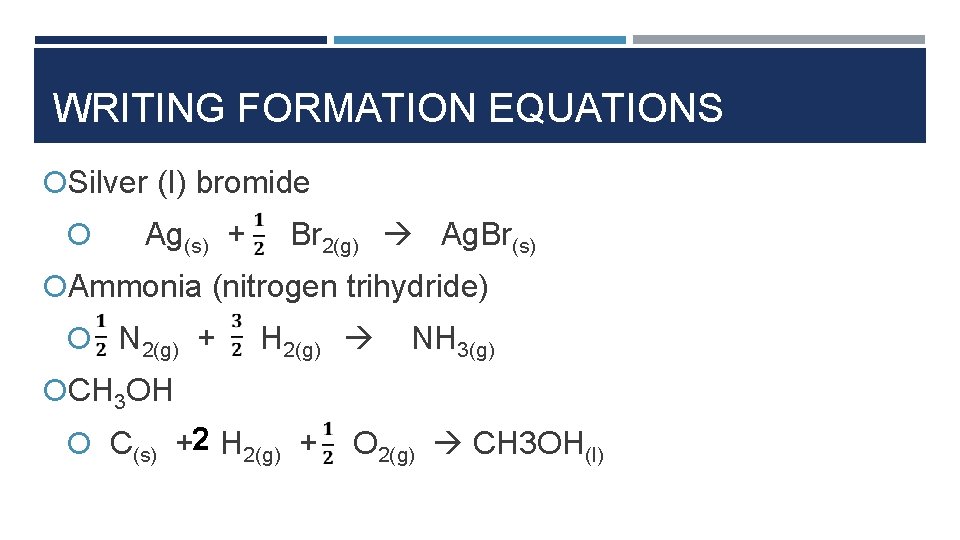
WRITING FORMATION EQUATIONS Silver (I) bromide Ag(s) + Br 2(g) Ag. Br(s) Ammonia (nitrogen trihydride) N 2(g) + H 2(g) NH 3(g) CH 3 OH C(s) +2 H 2(g) + O 2(g) CH 3 OH(l)
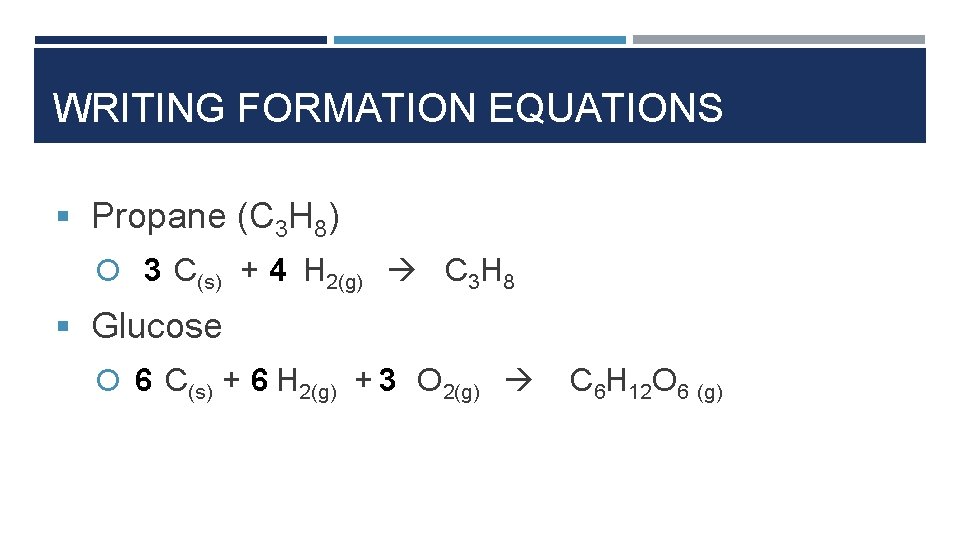
WRITING FORMATION EQUATIONS § Propane (C 3 H 8) 3 C(s) + 4 H 2(g) C 3 H 8 § Glucose 6 C(s) + 6 H 2(g) + 3 O 2(g) C 6 H 12 O 6 (g)
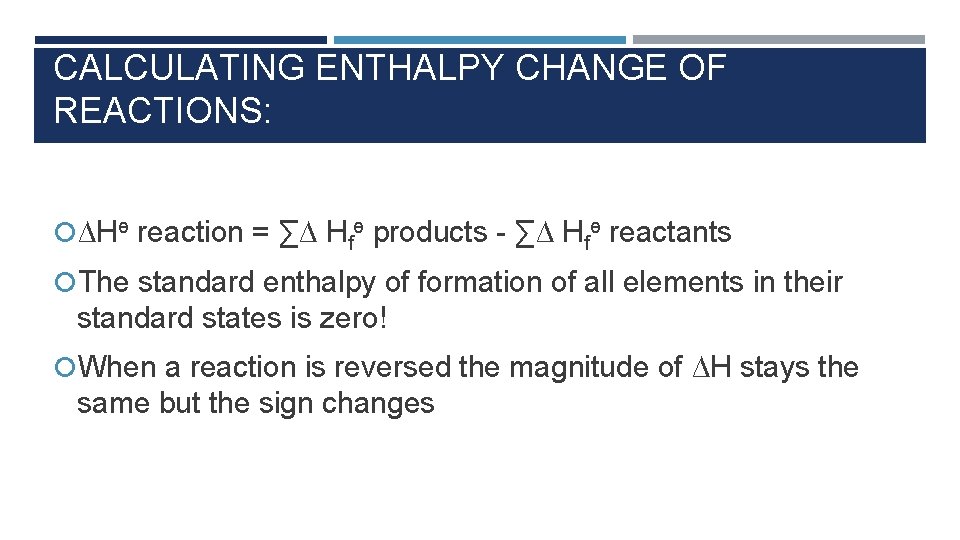
CALCULATING ENTHALPY CHANGE OF REACTIONS: ∆Hө reaction = ∑∆ Hfө products - ∑∆ Hfө reactants The standard enthalpy of formation of all elements in their standard states is zero! When a reaction is reversed the magnitude of ∆H stays the same but the sign changes
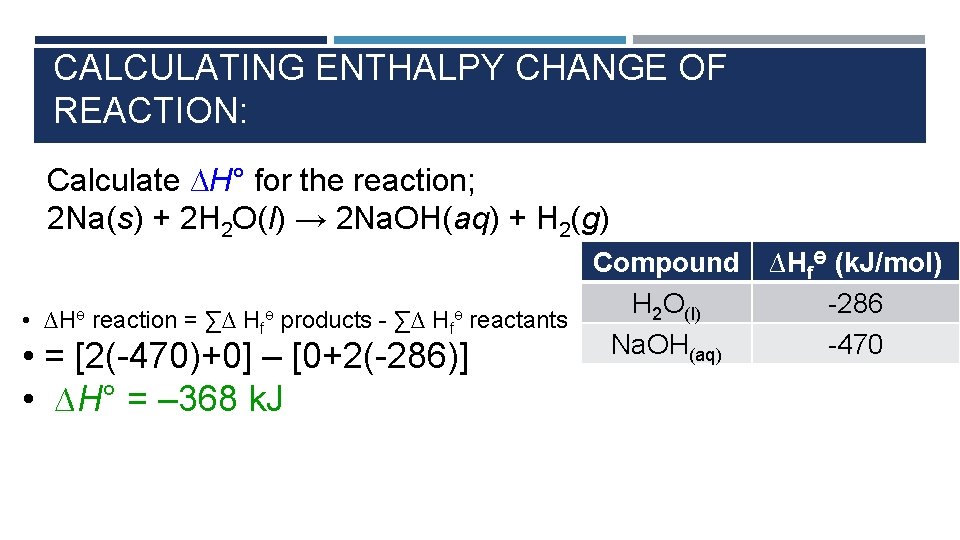
CALCULATING ENTHALPY CHANGE OF REACTION: Calculate ∆H° for the reaction; 2 Na(s) + 2 H 2 O(l) → 2 Na. OH(aq) + H 2(g) Compound H 2 O(l) • ∆Hө reaction = ∑∆ Hfө products - ∑∆ Hfө reactants Na. OH(aq) • = [2(-470)+0] – [0+2(-286)] • ∆H° = – 368 k. J ∆HfӨ (k. J/mol) -286 -470
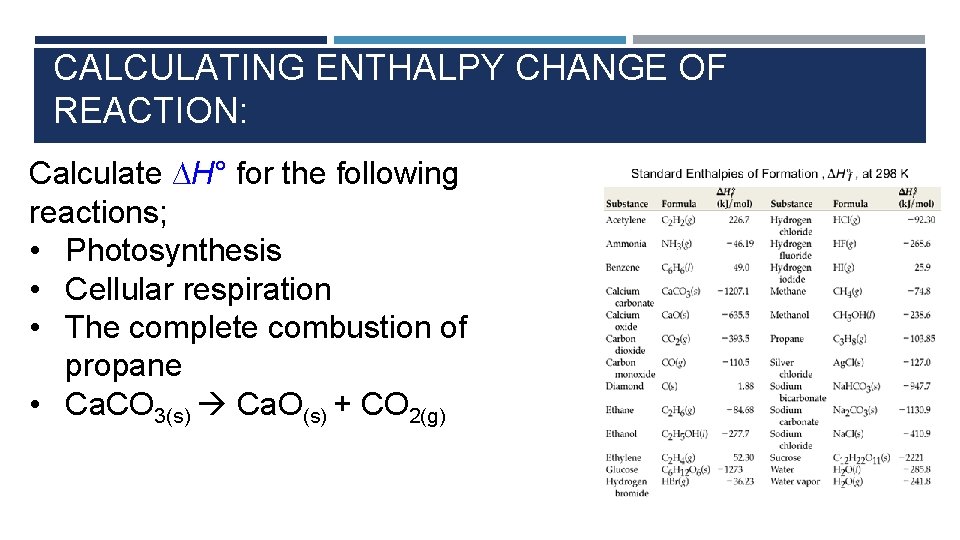
CALCULATING ENTHALPY CHANGE OF REACTION: Calculate ∆H° for the following reactions; • Photosynthesis • Cellular respiration • The complete combustion of propane • Ca. CO 3(s) Ca. O(s) + CO 2(g)
- Slides: 10