48 Atoms are the smallest form of elements



- Slides: 9

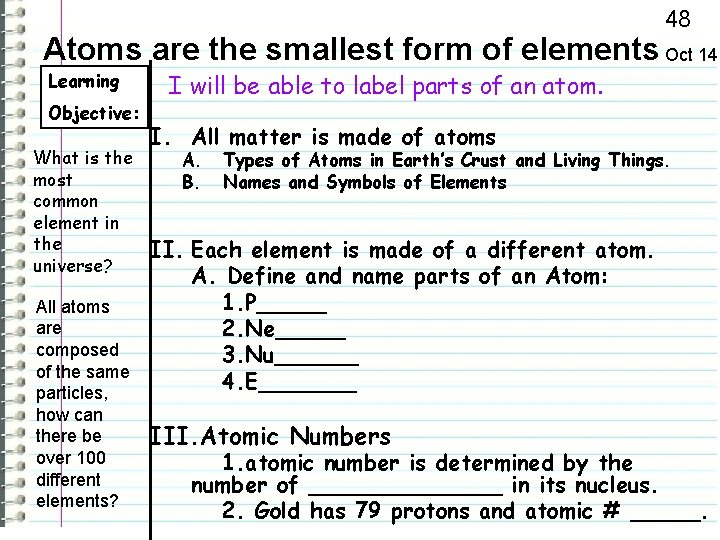
48 Atoms are the smallest form of elements Oct 14 Learning Objective: What is the most common element in the universe? All atoms are composed of the same particles, how can there be over 100 different elements? I will be able to label parts of an atom. I. All matter is made of atoms A. B. Types of Atoms in Earth’s Crust and Living Things. Names and Symbols of Elements II. Each element is made of a different atom. A. Define and name parts of an Atom: 1. P_____ 2. Ne_____ 3. Nu______ 4. E_______ III. Atomic Numbers 1. atomic number is determined by the number of _______ in its nucleus. 2. Gold has 79 protons and atomic # _____.

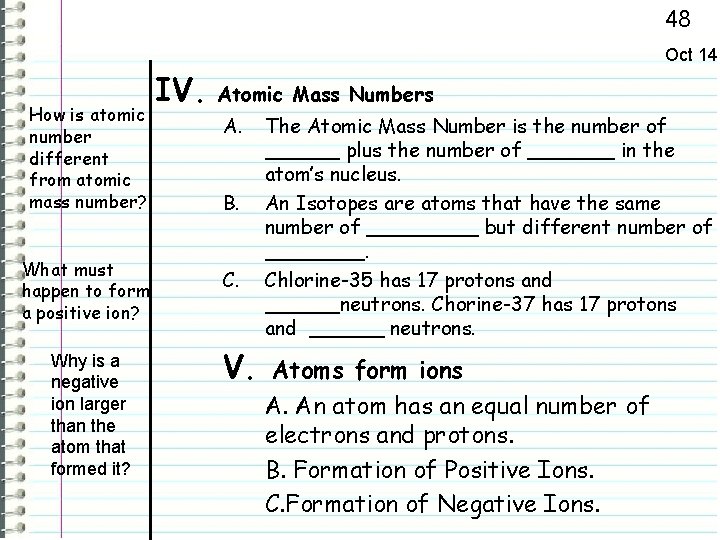
48 Oct 14 How is atomic number different from atomic mass number? What must happen to form a positive ion? Why is a negative ion larger than the atom that formed it? IV. Atomic Mass Numbers A. B. C. V. The Atomic Mass Number is the number of ______ plus the number of _______ in the atom’s nucleus. An Isotopes are atoms that have the same number of _____ but different number of ____. Chlorine-35 has 17 protons and ______neutrons. Chorine-37 has 17 protons and ______ neutrons. Atoms form ions A. An atom has an equal number of electrons and protons. B. Formation of Positive Ions. C. Formation of Negative Ions.

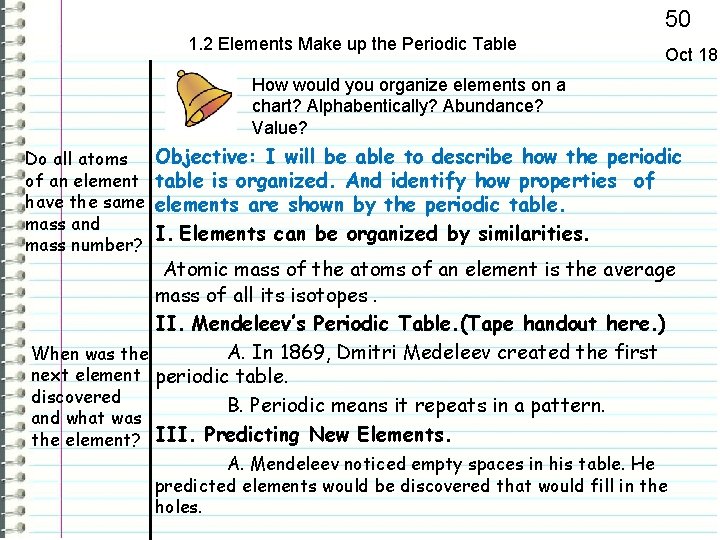
50 1. 2 Elements Make up the Periodic Table Oct 18 How would you organize elements on a chart? Alphabentically? Abundance? Value? Do all atoms of an element have the same mass and mass number? Objective: I will be able to describe how the periodic table is organized. And identify how properties of elements are shown by the periodic table. I. Elements can be organized by similarities. Atomic mass of the atoms of an element is the average mass of all its isotopes. II. Mendeleev’s Periodic Table. (Tape handout here. ) A. In 1869, Dmitri Medeleev created the first When was the next element periodic table. discovered B. Periodic means it repeats in a pattern. and what was the element? III. Predicting New Elements. A. Mendeleev noticed empty spaces in his table. He predicted elements would be discovered that would fill in the holes.

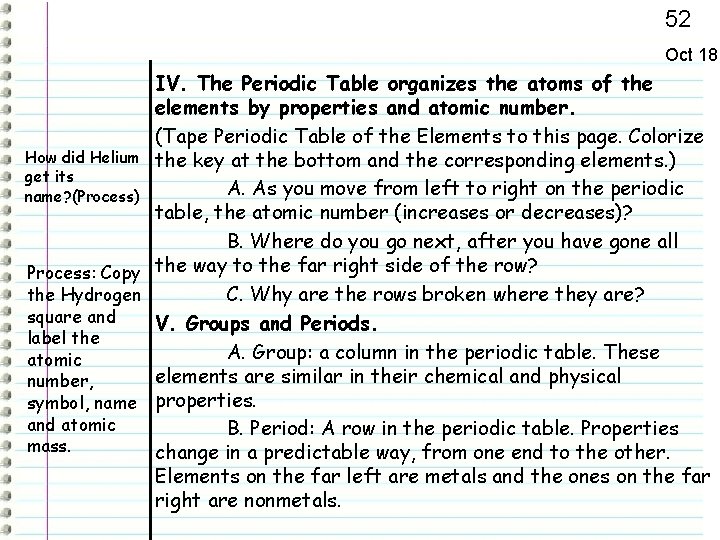
52 Oct 18 IV. The Periodic Table organizes the atoms of the elements by properties and atomic number. (Tape Periodic Table of the Elements to this page. Colorize How did Helium the key at the bottom and the corresponding elements. ) get its A. As you move from left to right on the periodic name? (Process) table, the atomic number (increases or decreases)? B. Where do you go next, after you have gone all Process: Copy the way to the far right side of the row? the Hydrogen C. Why are the rows broken where they are? square and V. Groups and Periods. label the A. Group: a column in the periodic table. These atomic elements are similar in their chemical and physical number, symbol, name properties. and atomic B. Period: A row in the periodic table. Properties mass. change in a predictable way, from one end to the other. Elements on the far left are metals and the ones on the far right are nonmetals.

52 Oct 18 VI. Trends I the Periodic Table. A. Which group easily loses an electron to form a positive ion? How did Helium B. Which group do not normally form an ion? get its C. Which side of the chart has the largest name? (Process) atoms? D. Where on the periodic table do you find the Process: Copy densest atoms? the Hydrogen E. Where would you find the largest atom on the square and chart? label the atomic number, symbol, name and atomic mass. (Process: Questions 1 -6, page 23)

50 1. 3 The Periodic Table is a map of the elements Oct 18 What are some of the properties and traits of elements that are shared? Is there a relationship between the property and the positioin on the periodic table? Do all atoms of an element have the same mass and mass number? Objective: I will be able to classify elements as metals, nonmetals, and metalloids. I will be able to list the different groups of elements. I will be able to explain what a radioactive element is. I. Elements can be organized by similarities. Atomic mass of the atoms of an element is the average mass of all its isotopes. When was the II. Mendeleev’s Periodic Table. (Tape handout here. ) next element A. In 1869, Dmitri Medeleev created the first discovered periodic table. and what was B. Periodic means it repeats in a pattern. the element? III. Predicting New Elements. A. Mendeleev noticed empty spaces in his table. He predicted elements would be discovered that would fill in the holes.


