4 WAYS to SHOW the Electron ConfigurationElectron arrangement

























![Shorthand Configuration Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] 4 Shorthand Configuration Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] 4](https://slidetodoc.com/presentation_image_h2/cc9df73e46b622481a7df68068a083ad/image-27.jpg)








![32 Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d 32 Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d](https://slidetodoc.com/presentation_image_h2/cc9df73e46b622481a7df68068a083ad/image-36.jpg)






![Stability • Electron Configuration Exceptions – Copper EXPECT: [Ar] 4 s 2 3 d Stability • Electron Configuration Exceptions – Copper EXPECT: [Ar] 4 s 2 3 d](https://slidetodoc.com/presentation_image_h2/cc9df73e46b622481a7df68068a083ad/image-43.jpg)
![Stability • Electron Configuration Exceptions – Chromium EXPECT: [Ar] 4 s 2 3 d Stability • Electron Configuration Exceptions – Chromium EXPECT: [Ar] 4 s 2 3 d](https://slidetodoc.com/presentation_image_h2/cc9df73e46b622481a7df68068a083ad/image-44.jpg)







- Slides: 51

4 WAYS to SHOW the Electron Configuration(Electron arrangement) Objectives: Learn how to show Electron configuration using: 1. 2. 3. 4. Using Aufbau Energy Diagrams Orbital Diagrams Long hand Electron configuration Short-hand Electron configuration

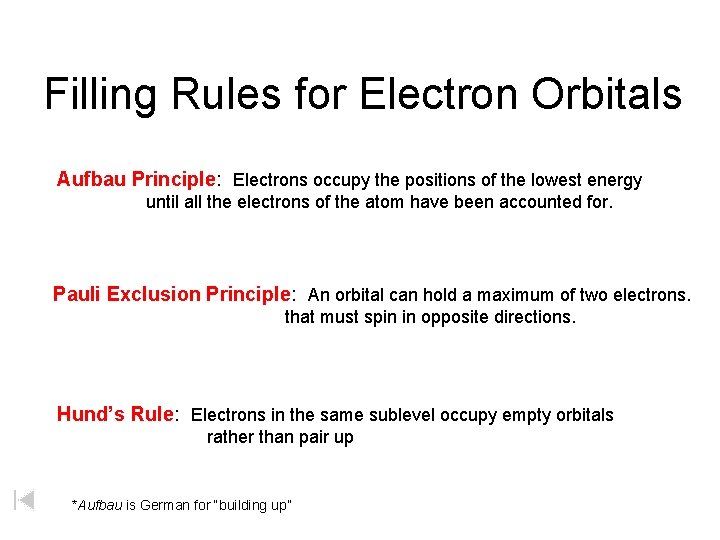
Filling Rules for Electron Orbitals Aufbau Principle: Electrons occupy the positions of the lowest energy until all the electrons of the atom have been accounted for. Pauli Exclusion Principle: An orbital can hold a maximum of two electrons. that must spin in opposite directions. Hund’s Rule: Electrons in the same sublevel occupy empty orbitals rather than pair up *Aufbau is German for “building up”

Periodic Patterns s 1 2 3 4 5 6 7 p 1 s 2 s f 2 p 3 s d (n-1) 4 s 3 d 4 p 5 s 4 d 5 p 6 s 5 d 6 p 7 s 6 d 7 p 6 (n-2) 7 3 p 4 f 5 f 1 s

Order in which subshells are filled with electrons (AUFBAU) 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f 5 s 5 p 5 d 5 f 6 s 6 p 6 d 7 s 2 2 6 2 10 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d …

4 f Sublevels 4 d Energy 6 d 5 f 7 s 6 p 5 d 4 f 6 s 5 p 4 d 5 s 4 p 3 d 4 s 3 p 6 d 7 s 6 s 5 p 5 s 4 p 4 s 5 d 4 f n=3 3 d 2 p 3 s 2 p 2 s 4 p 3 d 4 s 3 p 3 s 4 d 3 p 3 s 2 p 2 s 6 p 5 f Energy n=4 n=2 2 s 1 s 1 s n=1 1 s

4 f Sublevels 4 d s p s d p s n=4 f d p Energy s n=3 4 p 3 d 4 s 3 p 3 s 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 10… 2 p n=2 2 s n=1 1 s

Aufbau Energy Level Diagram of an Atom 6 s 6 p 5 d 4 f 32 5 s 5 p 4 d 18 4 s 4 p 3 d 18 Arbitrary Energy Scale 3 s 3 p 8 2 s 2 p 8 1 s 2 NUCLEUS O’Connor, Davis, Mac. Nab, Mc. Clellan, CHEMISTRY Experiments and Principles 1982, page 177

Electron capacities Copyright © 2006 Pearson Benjamin Cummings. All rights reserved.

2 Copyright © 2007 Pearson Benjamin Cummings. All rights reserved. 8 8 32 32 18 18

General Rules 6 d Aufbau Principle 7 s 6 p 5 d – Electrons fill the lowest energy orbitals first. 6 s 4 d 3 p 7 s 5 f 6 p 5 d 6 s 5 p 5 s 4 p 4 s 6 d 4 f 5 p Energy – “Lazy Tenant Rule” 5 f 4 d 5 s 3 d 4 p 3 d 4 s 3 p 3 s 3 s 2 p 2 p 2 s 2 s 1 s 1 s Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem 4 f

Order in which subshells are filled with electrons (AUFBAU) 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f 5 s 5 p 5 d 5 f 6 s 6 p 6 d 7 s 2 2 6 2 10 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d …

LET’s WATCH this SIMPLE VIDEO on how to use the AUFBAU diagram To WRITE ELECTRON configurations VIDEO LINK “ LET’S DO IT!” If you believe you haven’t understood Anything so far here is a simple video. However, the electron config is WRONG For Lithium when she shows you. It is Li 1 s 2 2 s 1

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Hydrogen 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La H = 1 s 1

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Helium 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La He = 1 s 2

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Lithium 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La Li = 1 s 22 s 1

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Carbon 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La C = 1 s 22 p 2

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Nitrogen 4 f Bohr Model N 2 s 2 p Hund’s Rule “maximum number of unpaired orbitals”. 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La N = 1 s 22 p 3

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Fluorine 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La F = 1 s 22 p 5

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Aluminum 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La Al = 1 s 22 p 63 s 23 p 1

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Argon 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La Ar = 1 s 22 p 63 s 23 p 6

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Iron 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe = 1 s 22 p 63 s 23 p 64 s 23 d 6 Fe La

Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Lanthanum 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La La = 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 66 s 25 d 1

Electron Configurations Orbital Filling Element 1 s 2 s 2 px 2 py 2 pz 3 s Electron Configuration H 1 s 1 He 1 s 2 C NOT CORRECT 1 s 22 s 1 Violates Hund’s Rule 1 s 22 p 2 N 1 s 22 p 3 O 1 s 22 p 4 F 1 s 22 p 5 Ne 1 s 22 p 6 Na 1 s 22 p 63 s 1 Li

Electron Configurations Orbital Filling Element 1 s 2 s 2 px 2 py 2 pz 3 s Electron Configuration H 1 s 1 He 1 s 2 Li 1 s 22 s 1 C 1 s 22 p 2 N 1 s 22 p 3 O 1 s 22 p 4 F 1 s 22 p 5 Ne 1 s 22 p 6 Na 1 s 22 p 63 s 1

Shorthand Configuration A neon's electron configuration (1 s 22 p 6) B third energy level [Ne] 3 s 1 C D one electron in the s orbital shape Na = [1 s 22 p 6] 3 s 1 electron configuration
![Shorthand Configuration Element symbol Electron configuration Ca Ar 4 s 2 V Ar 4 Shorthand Configuration Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] 4](https://slidetodoc.com/presentation_image_h2/cc9df73e46b622481a7df68068a083ad/image-27.jpg)
Shorthand Configuration Element symbol Electron configuration Ca [Ar] 4 s 2 V [Ar] 4 s 2 3 d 3 F [He] 2 s 2 2 p 5 Ag [Kr] 5 s 2 4 d 9 I [Kr] 5 s 2 4 d 10 5 p 5 Xe [Kr] 5 s 2 4 d 10 5 p 6 Fe Sg 22 p 64 s [He] 2 s[Ar] 3 s 223 d 3 p 664 s 23 d 6 [Rn] 7 s 2 5 f 14 6 d 4

General Rules • Pauli Exclusion Principle – Each orbital can hold TWO electrons with opposite spins. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem Wolfgang Pauli

General Rules • Hund’s Rule – Within a sublevel, place one electron per orbital before pairing them. – “Empty Bus Seat Rule” WRONG RIGHT Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

O Notation 15. 9994 • Orbital Diagram O 8 e- 1 s 2 s • Electron Configuration 2 2 4 1 s 2 s 2 p Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem 8 2 p

Notation S 32. 066 • Longhand Configuration S 16 e 6 2 2 2 1 s 2 s 2 p 3 s Core Electrons S 16 e 4 3 p Valence Electrons • Shorthand Configuration 2 4 [Ne] 3 s 3 p Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem 16

Periodic Patterns s 1 2 3 4 5 6 7 p 1 s 2 s f 2 p 3 s d (n-1) 4 s 3 d 4 p 5 s 4 d 5 p 6 s 5 d 6 p 7 s 6 d 7 p 6 (n-2) 7 3 p 4 f 5 f 1 s

Periodic Patterns • Period # – energy level (subtract for d & f) • A/B Group # – total # of valence e- • Column within sublevel block – # of e- in sublevel Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Periodic Patterns • Example - Hydrogen 1 1 s 1 st Period 1 st column of s-block Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Periodic Patterns • Shorthand Configuration – Core electrons: • Go up one row and over to the Noble Gas. – Valence electrons: • On the next row, fill in the # of e- in each sublevel. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
![32 Periodic Patterns Example Germanium Ar 2 4 s 10 3 d 32 Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d](https://slidetodoc.com/presentation_image_h2/cc9df73e46b622481a7df68068a083ad/image-36.jpg)
32 Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem 2 4 p Ge 72. 61

Stability • Full energy level • Full sublevel (s, p, d, f) • Half-full sublevel Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

The Octet Rule Atoms tend to gain, lose, or share electrons until they have eight valence electrons. This fills the valence shell and tends to give the atom the stability of the inert gasses. 8 ONLY s- and p-orbitals are valence electrons.

THIS SLIDE IS ANIMATED IN FILLING ORDER 2. PPT H = 1 s 1 1 s He = 1 s 2 1 s Li = 1 s 2 2 s 1 1 s 2 s 2 px 2 py 2 pz Be = 1 s 2 2 s 2 C = 1 s 2 2 p 2 S = 1 s 2 2 p 4 3 s 3 px 3 py 3 pz

26 electrons. Iron has ___ Fe = 1 s 1 2 s 22 p 63 s 23 p 64 s 23 d 6 1 s 2 px 2 py 2 pz 2 s 3 s 3 px 3 py 3 pz 6 s 6 p 4 s 5 d 3 d 3 d 3 d 4 f 32 5 s e- eee- e- e- +26 e- e- ee- e- e- 4 s 4 p 3 d e- ee- e- e- 4 d 18 e- e- 5 p 18 Arbitrary Energy Scale 3 s 3 p 8 ee- 2 s 2 p 8 1 s 2 NUCLEUS 3 d 3 d

Energy Level Diagram of a Many-Electron Atom 6 s 6 p 5 d 4 f 32 5 s 5 p 4 d 18 4 s 4 p 3 d 18 Arbitrary Energy Scale 3 s 3 p 8 2 s 2 p 8 1 s 2 NUCLEUS O’Connor, Davis, Mac. Nab, Mc. Clellan, CHEMISTRY Experiments and Principles 1982, page 177

Maximum Number of Electrons In Each Sublevel Number of Orbitals Maximum Number of Electrons s 1 2 p 3 6 d 5 10 f 7 14 Le. May Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 146
![Stability Electron Configuration Exceptions Copper EXPECT Ar 4 s 2 3 d Stability • Electron Configuration Exceptions – Copper EXPECT: [Ar] 4 s 2 3 d](https://slidetodoc.com/presentation_image_h2/cc9df73e46b622481a7df68068a083ad/image-43.jpg)
Stability • Electron Configuration Exceptions – Copper EXPECT: [Ar] 4 s 2 3 d 9 ACTUALLY: [Ar] 4 s 1 3 d 10 – Copper gains stability with a full d-sublevel. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
![Stability Electron Configuration Exceptions Chromium EXPECT Ar 4 s 2 3 d Stability • Electron Configuration Exceptions – Chromium EXPECT: [Ar] 4 s 2 3 d](https://slidetodoc.com/presentation_image_h2/cc9df73e46b622481a7df68068a083ad/image-44.jpg)
Stability • Electron Configuration Exceptions – Chromium EXPECT: [Ar] 4 s 2 3 d 4 ACTUALLY: [Ar] 4 s 1 3 d 5 – Chromium gains stability with a half-full d -sublevel. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Electron Filling in Periodic Table s s p 1 2 d 3 4 K 4 s 1 Ca 4 s 2 Sc 3 d 1 Ti 3 d 2 4 f Energy n=4 n=3 n=2 n=1 V 3 d 3 Cr 3 d 54 Mn 3 d 5 Fe 3 d 6 Co 3 d 7 Ni 3 d 8 Cu 9 3 d 3 d 10 Cr Cu 4 s 13 d 5 4 s 13 d 10 Zn 3 d 10 Ga 4 p 1 Ge 4 p 2 As 4 p 3 Se 4 p 4 Br 4 p 5 Kr 4 p 6 4 d 4 p 3 d 4 s 3 p 3 s Cr 4 s 13 d 5 4 s 3 d 2 p 2 s Cu 1 s 4 s 13 d 10 4 s 3 d

Stability • Ion Formation – Atoms gain or lose electrons to become more stable. – Isoelectronic with the Noble Gases. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Stability • Ion Electron Configuration – Write the e- configuration for the closest Noble Gas • EX: Oxygen ion O 2 - Ne O 2 - 10 e- [He] 2 s 2 2 p 6 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Orbital Diagrams for Nickel 2 1 s 2 s 2 6 2 p 2 3 s 6 3 p 2 4 s 8 3 d Excited State 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 9 Pauli Exclusion 1 s 2 s 2 p 3 s 3 p 4 s 3 d Hund’s Rule 1 s 2 s 2 p 3 s 3 p 4 s 3 d 28 Ni 58. 6934

Orbital Diagrams for Nickel 2 1 s 2 s 2 6 2 p 2 3 s 6 3 p 2 8 4 s 3 d Excited State 1 s 2 2 p 6 2 3 s 3 p 6 4 s 1 3 d 9 VIOLATES Pauli Exclusion 1 s 2 s 2 p 3 s 3 p 4 s 3 d VIOLATES Hund’s Rule 1 s 2 s 2 p 3 s 3 p 4 s 3 d 28 Ni 58. 6934

Write out the complete electron configuration for the following: 1) An atom of nitrogen 2) An atom of silver POP QUIZ 3) An atom of uranium (shorthand) Fill in the orbital boxes for an atom of nickel (Ni) 1 s 2 s 2 p 3 s 3 p 4 s 3 d Which rule states no two electrons can spin the same direction in a single orbital? Extra credit: Draw a Bohr model of a Ti 4+ cation. Ti 4+ is isoelectronic to Argon.

Answer Key Write out the complete electron configuration for the following: 1) An atom of nitrogen 1 s 22 p 3 2) An atom of silver 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 9 3) An atom of uranium (shorthand) [Rn]7 s 26 d 15 f 3 Fill in the orbital boxes for an atom of nickel (Ni) 1 s 2 s 2 p 3 s 3 p 4 s 3 d Which rule states no two electrons can spin the same direction in a single orbital? Pauli exclusion principle Extra credit: Draw a Bohr model of a Ti 4+ cation. Ti 4+ is isoelectronic to Argon. n= 22+ n