4 th KitasatoHarvard Symposium Summary and Conclusions Stephen












- Slides: 13

4 th Kitasato-Harvard Symposium: Summary and Conclusions Stephen Lagakos Harvard School of Public Health

This year’s goals • Advance Global Drug Development by discussion of – emerging trends – technology updates – novel paradigms

Organization of Discussion into 5 Sessions • Global programs: Is there a Reality? • Regulatory updates and clinical trends • Practical use of ICH guidelines for bridging and global trials • Use of genomics, preteomics, and metabolomics to enhance evaluation of drugs • Other novel technology updates: modeling, biomarkers, surrogates, and data mining

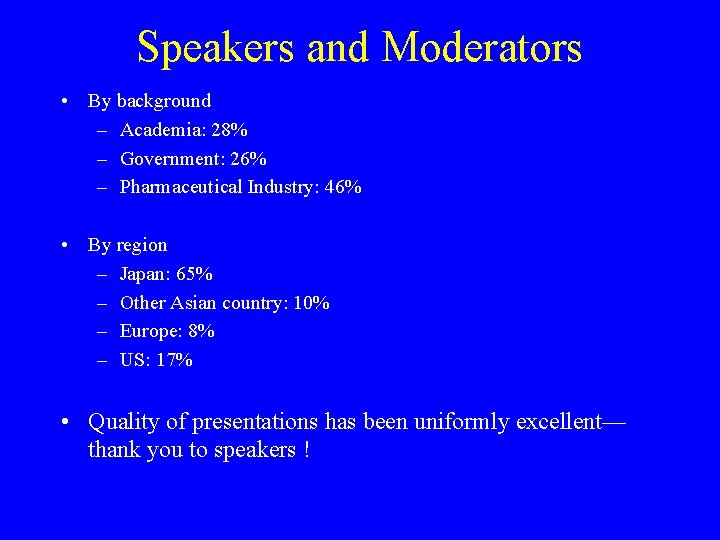
Speakers and Moderators • By background – Academia: 28% – Government: 26% – Pharmaceutical Industry: 46% • By region – Japan: 65% – Other Asian country: 10% – Europe: 8% – US: 17% • Quality of presentations has been uniformly excellent— thank you to speakers !

Sessions 1/2: Global Trials & Regulatory Updates • Recent changes at MHLW will hopefully lead to more expedited reviews • Several important lessons learned from doing global trials – Importance of standardization of endpoints, definitions, protocols – Need to establish a network among regulatory agencies to facilitate data sharing and review (common? ) and implementation of results – Additional complexities but many advantages in terms of getting more definitive answers and getting results sooner

Numerous challenges for Japan in doing clinical trials – Costs is very high, and appears to be due mainly to clinical costs – Lack of availability of enough sites – Awareness of and desire by public to participate in clinical research less than in some other regions • Reasons unclear: but related to less developed infrastructure in academia/medical centers compared to other countries (eg, US), where clinical research within academia is recognized and rewarded

Global Trials • Who has responsibility to increase capacity and conduct of trials in Japan: • • • Government? Industry? Academia? Þ “Yes” to all ! -MHLW, in particular, must do what it can to promote conduct of more trials in Japan, without lowering standards of review—a difficult challenge. -Number of medical and statistical reviewers in MHLW is still too small. Additional resources needed, and importance of these positions needs to be better recognized by scientific community.

Increasing Capacity in Japan • One approach: large scale CT network – Ideal if this develops capacity at academic/medical centers – Role of Japanese Med. Association a bit unclear (to me). In US, much responsibility rests within academia through governmental funding of academic cooperative groups of academic units. Scientific leadership from academic researchers, not government – What is best for Japan overall?

Global versus Bridging Studies • Global trials will, in general, get answers sooner and more definitively • Simultaneous evaluation of a drug in several regions can avoid ethical dilemmas – e. g. : if a new drug appears to be very efficacious in a placebo-controlled trial in the US, is it still ethical to use a placebo control if the study is later done in Japan?

Session 3: Practical Use of ICH Guidelines for Bridging/Global Trials • ICH advances will further encourage & facilitate multinational studies and bridging studies • Increased experience with bridging studies will likely reduce the need for these (Bob O’Neill) • Use of surrogate endpoints for bridging very attractive; use depends on acceptance of the surrogate by the scientific community. Examples: – Many vaccines: immune response – HIV/AIDS: viral load – Osteoporosis: bone mineral density

Sessions 4 and 5: How can we use “omics” and other new technologies? • Excellent examples of how new technologies can be useful in all aspects of drug development • Most believe that future use of genomic information will increase and offers promise of eventually developing ‘individualized therapy’ • Use of genetic data also poses new challenges, both scientific (due to high dimensionality) and societal (trust by the public; confidentiality). • Other novel technologies (visual, acoustic, modeling) offer promise for new and better endpoints to use in evaluating drugs

Final Comments • During the 4 years that this symposium has begun, there has been clear progress in discussing how we can move to more expedited evaluations of drugs – clearer notion of how to do bridging studies – recognition of the great potential of global trials – also a greater recognition of the challenges in doing multinational trials – coordination of regulatory agencies recognized as a critical step – Role of ‘omics’ more clearly understood and continues to offer great promise for future advances; other new technologies should allow use to refine outcomes and thereby better measure effects of drugs • A key challenge for Japan is how to enhance its ability to conduct large clinical trials

Final Comments • Goal of this symposium has to bring together persons with diverse backgrounds to share views express our differences in opinions in an open forum where we can learn from one another • We are reminded that the administrative and regulatory aspects streamlining drug development and evaluation are key to advancement of the science • Thank you for attending and we hope to see you next year !