4 Major Types of The 5 Reactions We




- Slides: 8

4 Major Types of The 5 Reactions

We can classify any reaction in the universe as 1 of 5 types based on the reactants and the products. We can use a general equation (using X, Y or Z to represent any element) to show what will happen overall. These 5 types are : Ø Synthesis Ø Decomposition Ø Single Displacement Ø Double Displacement Ø Complete Combustion

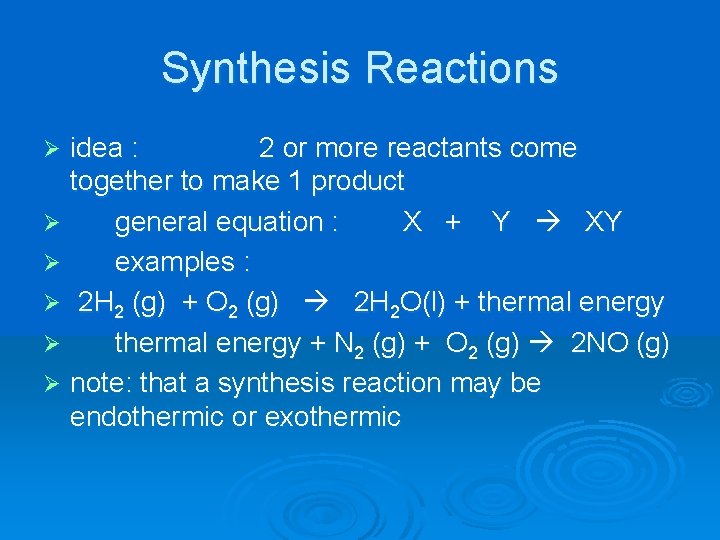
Synthesis Reactions idea : 2 or more reactants come together to make 1 product Ø general equation : X + Y XY Ø examples : Ø 2 H 2 (g) + O 2 (g) 2 H 2 O(l) + thermal energy Ø thermal energy + N 2 (g) + O 2 (g) 2 NO (g) Ø note: that a synthesis reaction may be endothermic or exothermic Ø

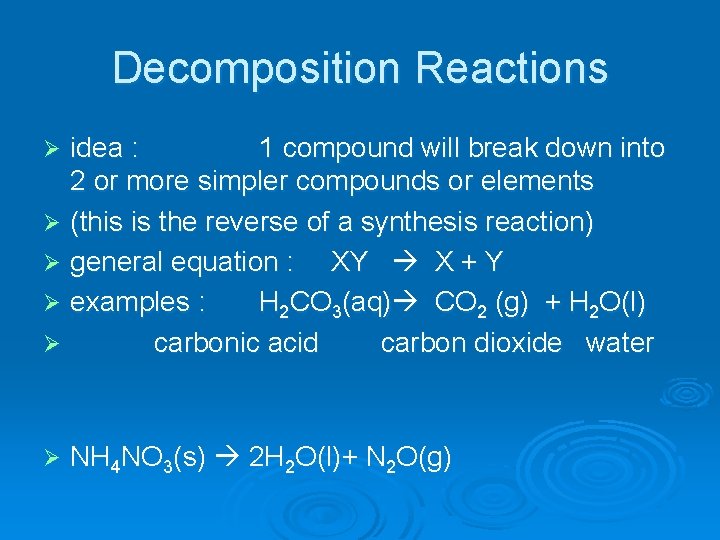
Decomposition Reactions idea : 1 compound will break down into 2 or more simpler compounds or elements Ø (this is the reverse of a synthesis reaction) Ø general equation : XY X + Y Ø examples : H 2 CO 3(aq) CO 2 (g) + H 2 O(l) Ø carbonic acid carbon dioxide water Ø Ø NH 4 NO 3(s) 2 H 2 O(l)+ N 2 O(g)

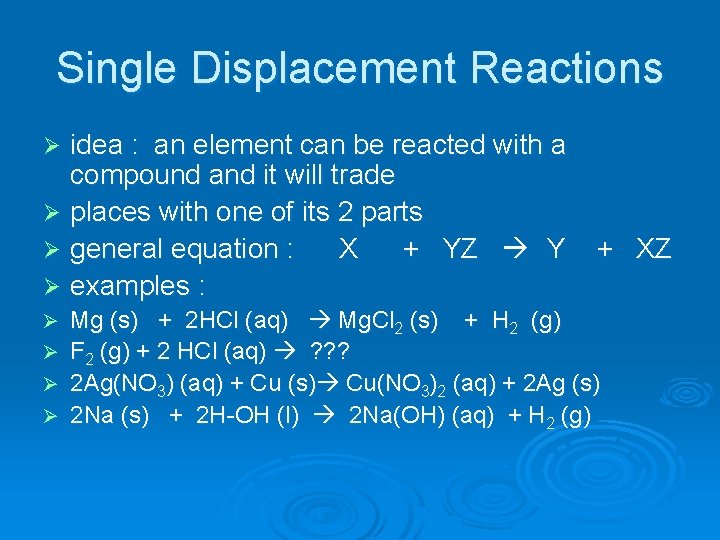
Single Displacement Reactions idea : an element can be reacted with a compound and it will trade Ø places with one of its 2 parts Ø general equation : X + YZ Y Ø examples : Ø Ø Ø + XZ Mg (s) + 2 HCl (aq) Mg. Cl 2 (s) + H 2 (g) F 2 (g) + 2 HCl (aq) ? ? ? 2 Ag(NO 3) (aq) + Cu (s) Cu(NO 3)2 (aq) + 2 Ag (s) 2 Na (s) + 2 H-OH (l) 2 Na(OH) (aq) + H 2 (g)

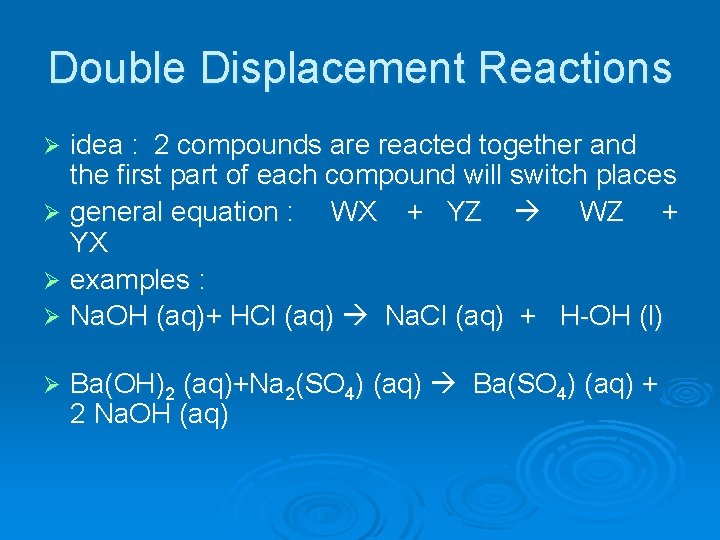
Double Displacement Reactions idea : 2 compounds are reacted together and the first part of each compound will switch places Ø general equation : WX + YZ WZ + YX Ø examples : Ø Na. OH (aq)+ HCl (aq) Na. Cl (aq) + H-OH (l) Ø Ø Ba(OH)2 (aq)+Na 2(SO 4) (aq) Ba(SO 4) (aq) + 2 Na. OH (aq)

Complete Combustion Ø Idea: the addition of a hydrocarbon to oxygen in the presence of a spark or flame will generate liquid water and carbon dioxide gas Ø General equation: Ø Cx. Hy +? O 2 (g) ? CO 2 (g) + y/2 H 2 O (l) Ø Example: C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l)

Practice Ø p. 121 4 -7 Ø p. 128 1 -4 Ø p. 138 1 -3