4 Major Types of Organic Macromolecules Found in








- Slides: 42

4 Major Types of Organic Macromolecules Found in Cells

Amoeba Sisters!

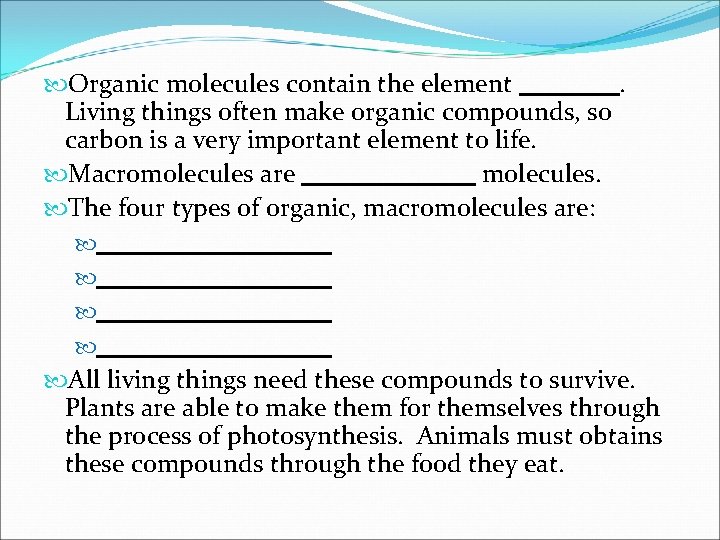
Organic molecules contain the element. Living things often make organic compounds, so carbon is a very important element to life. Macromolecules are molecules. The four types of organic, macromolecules are: All living things need these compounds to survive. Plants are able to make them for themselves through the process of photosynthesis. Animals must obtains these compounds through the food they eat.

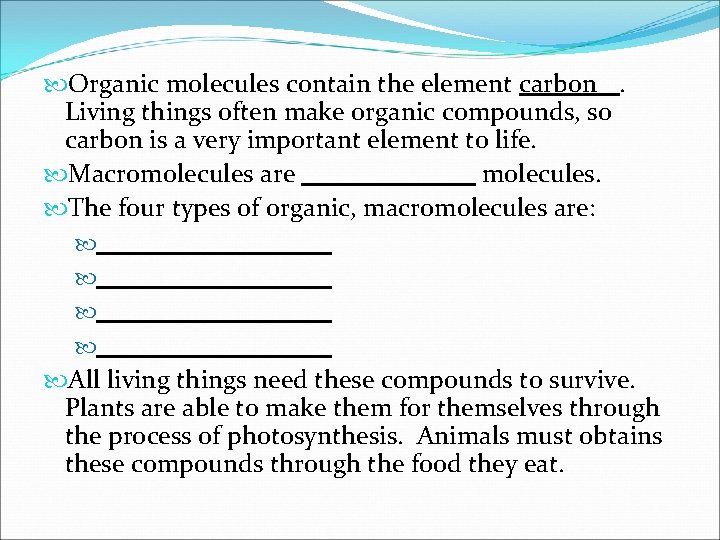
Organic molecules contain the element carbon. Living things often make organic compounds, so carbon is a very important element to life. Macromolecules are molecules. The four types of organic, macromolecules are: All living things need these compounds to survive. Plants are able to make them for themselves through the process of photosynthesis. Animals must obtains these compounds through the food they eat.

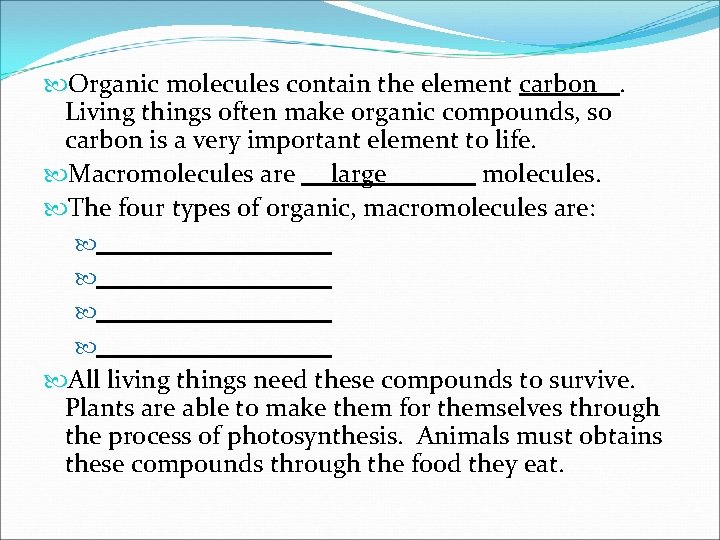
Organic molecules contain the element carbon. Living things often make organic compounds, so carbon is a very important element to life. Macromolecules are large molecules. The four types of organic, macromolecules are: All living things need these compounds to survive. Plants are able to make them for themselves through the process of photosynthesis. Animals must obtains these compounds through the food they eat.

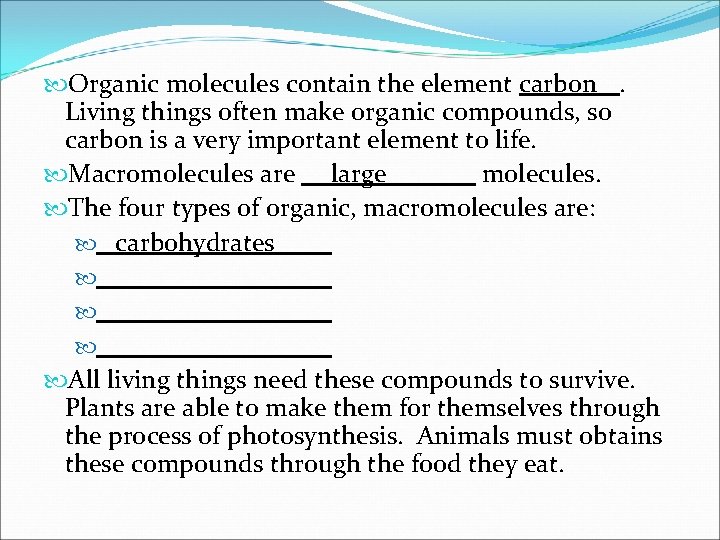
Organic molecules contain the element carbon. Living things often make organic compounds, so carbon is a very important element to life. Macromolecules are large molecules. The four types of organic, macromolecules are: carbohydrates All living things need these compounds to survive. Plants are able to make them for themselves through the process of photosynthesis. Animals must obtains these compounds through the food they eat.

Organic molecules contain the element carbon. Living things often make organic compounds, so carbon is a very important element to life. Macromolecules are large molecules. The four types of organic, macromolecules are: carbohydrates lipids All living things need these compounds to survive. Plants are able to make them for themselves through the process of photosynthesis. Animals must obtains these compounds through the food they eat.

Organic molecules contain the element carbon. Living things often make organic compounds, so carbon is a very important element to life. Macromolecules are large molecules. The four types of organic, macromolecules are: carbohydrates lipids proteins All living things need these compounds to survive. Plants are able to make them for themselves through the process of photosynthesis. Animals must obtains these compounds through the food they eat.

Organic molecules contain the element carbon. Living things often make organic compounds, so carbon is a very important element to life. Macromolecules are large molecules. The four types of organic, macromolecules are: carbohydrates lipids proteins nucleic acids All living things need these compounds to survive. Plants are able to make them for themselves through the process of photosynthesis. Animals must obtains these compounds through the food they eat.

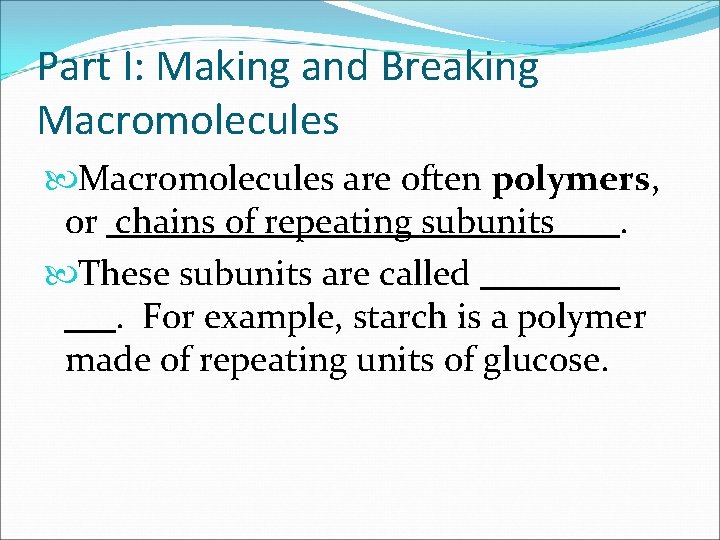
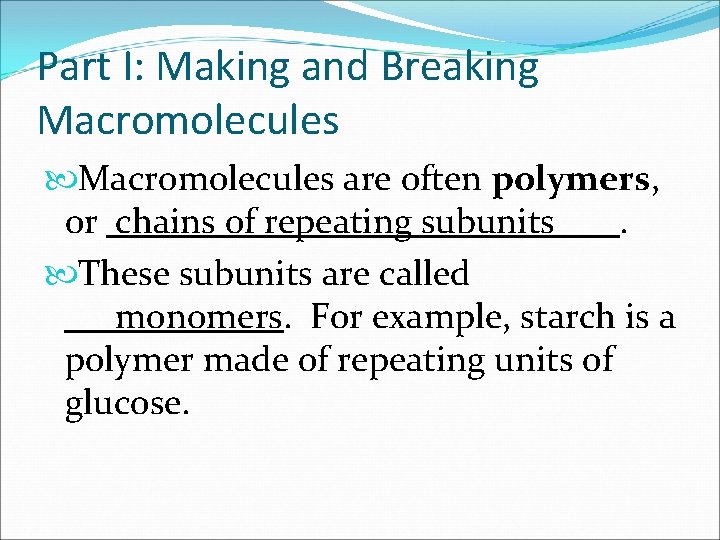
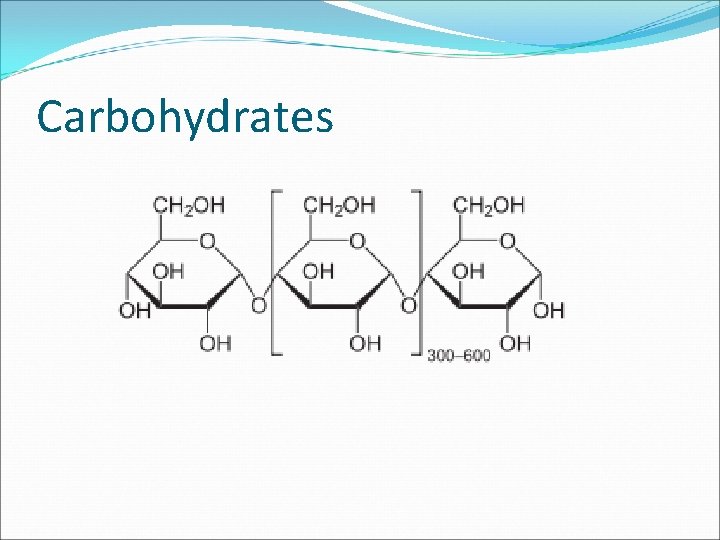
Part I: Making and Breaking Macromolecules are often polymers, or. These subunits are called. For example, starch is a polymer made of repeating units of glucose.

Part I: Making and Breaking Macromolecules are often polymers, or chains of repeating subunits. These subunits are called. For example, starch is a polymer made of repeating units of glucose.

Part I: Making and Breaking Macromolecules are often polymers, or chains of repeating subunits. These subunits are called monomers. For example, starch is a polymer made of repeating units of glucose.

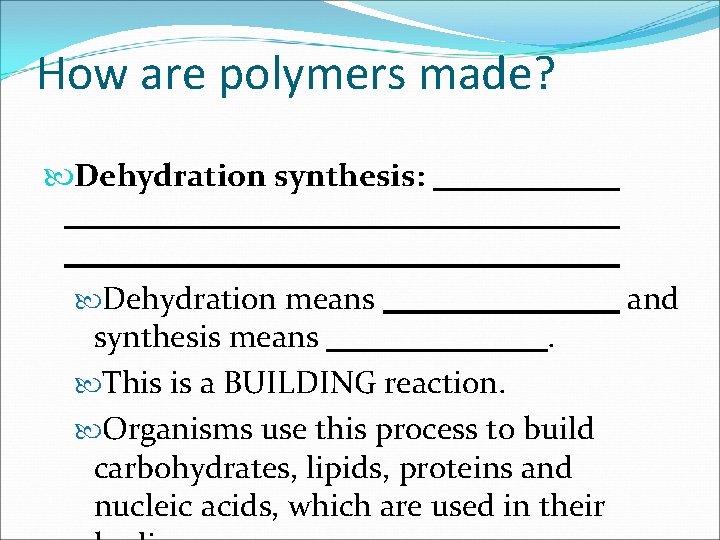
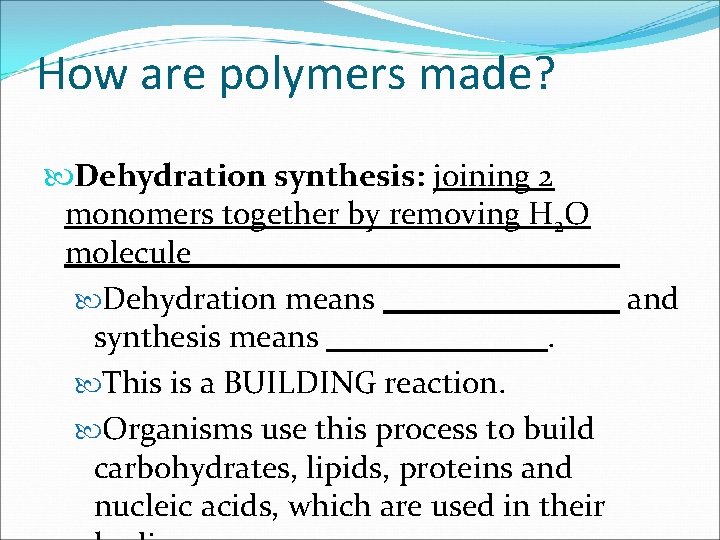
How are polymers made? Dehydration synthesis: Dehydration means synthesis means. This is a BUILDING reaction. Organisms use this process to build carbohydrates, lipids, proteins and nucleic acids, which are used in their and

How are polymers made? Dehydration synthesis: joining 2 monomers together by removing H 2 O molecule Dehydration means and synthesis means. This is a BUILDING reaction. Organisms use this process to build carbohydrates, lipids, proteins and nucleic acids, which are used in their

How are polymers made? Dehydration synthesis: joining 2 monomers together by removing H 2 O molecule Dehydration means to take out H 2 O and synthesis means. This is a BUILDING reaction. Organisms use this process to build carbohydrates, lipids, proteins and nucleic acids, which are used in their

How are polymers made? Dehydration synthesis: joining 2 monomers together by removing H 2 O molecule Dehydration means to take out H 2 O and synthesis means to build. This is a BUILDING reaction. Organisms use this process to build carbohydrates, lipids, proteins and nucleic acids, which are used in their

How are polymers broken down? Hydrolysis: Hydro means and lysis means This is a BREAKING reaction. This is what happens when animals digest food. Once we have monomers, we can reassemble them into polymers we can use by dehydration synthesis.

How are polymers broken down? Hydrolysis: breaking a monomer off a polymer Hydro means and lysis means This is a BREAKING reaction. This is what happens when animals digest food. Once we have monomers, we can reassemble them into polymers we can use by dehydration synthesis.

How are polymers broken down? Hydrolysis: breaking a monomer off a polymer Hydro means water and lysis means This is a BREAKING reaction. This is what happens when animals digest food. Once we have monomers, we can reassemble them into polymers we can use by dehydration synthesis.

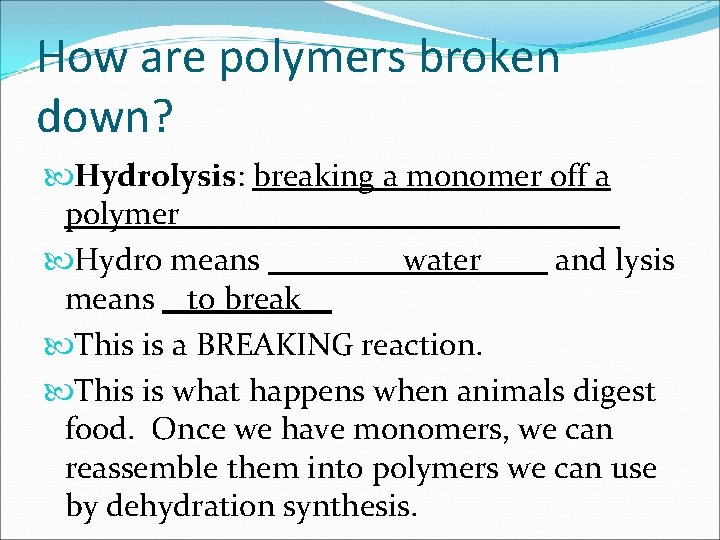
How are polymers broken down? Hydrolysis: breaking a monomer off a polymer Hydro means water and lysis means to break This is a BREAKING reaction. This is what happens when animals digest food. Once we have monomers, we can reassemble them into polymers we can use by dehydration synthesis.

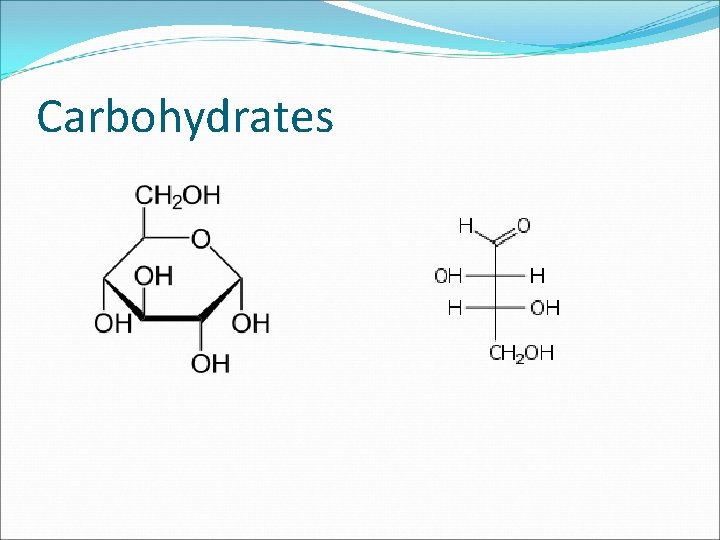
Carbohydrates Includes sugars and starches (basically anything that ends in –ose) Elements C, H, O Always will appear in a 1: 2: 1 ratio (twice as much hydrogen as carbon or oxygen) Building Blocks Monosaccharides, aka simple sugars Ex. Glucose, fructose, galactose

Carbohydrates

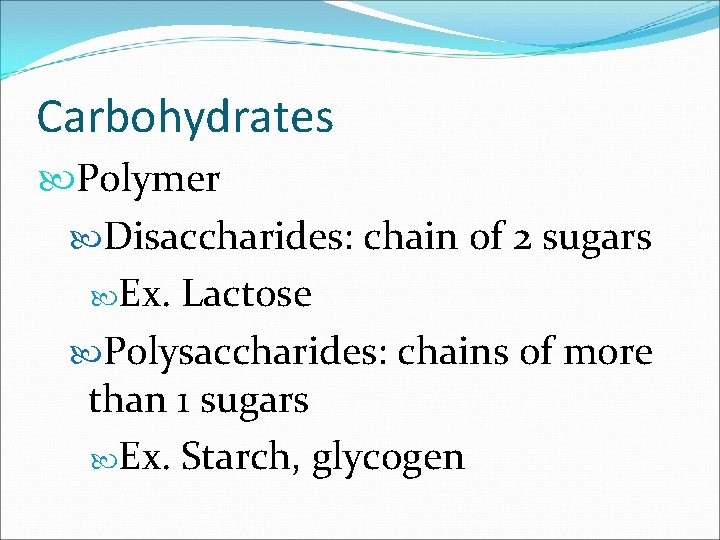
Carbohydrates Polymer Disaccharides: chain of 2 sugars Ex. Lactose Polysaccharides: chains of more than 1 sugars Ex. Starch, glycogen

Carbohydrates

Carbohydrates Functions Living things use carbohydrates as their main source of energy Simple sugars are used for quick energy because they break down very quickly Can also use for energy storage (glycogen in animals, cellulose in plants).

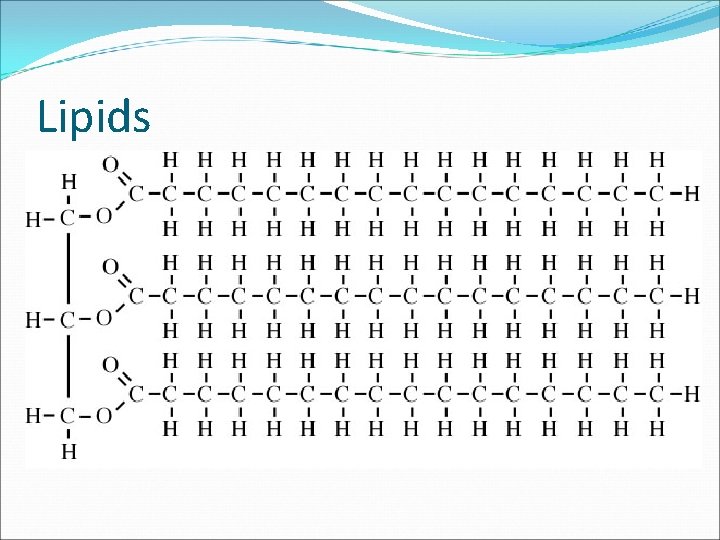
Lipids Hydrophobic, nonpolar molecules that come in many different forms Elements C, H, O Many more hydrogen atoms than oxygen atoms (much more than twice)

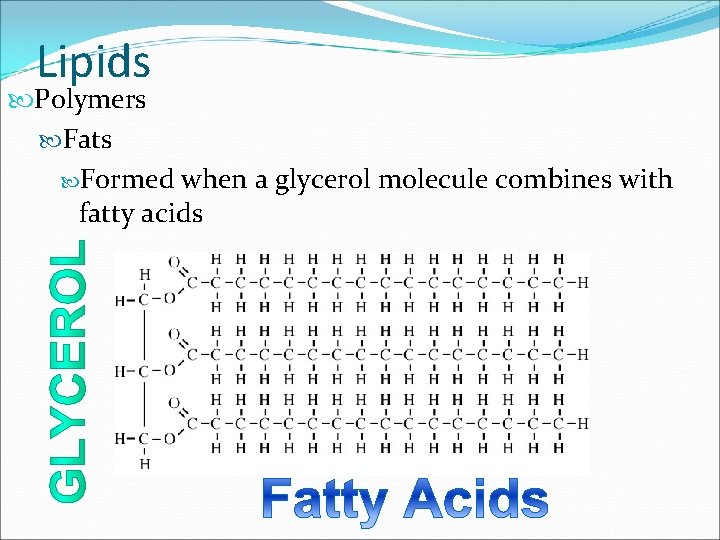
Lipids Building Blocks Do not have traditional building blocks that are all the same; they differ from lipid to lipid Ex. Glycerol molecule + 3 fatty acids = triglyceride

Lipids

Lipids Polymers Fats Formed when a glycerol molecule combines with fatty acids

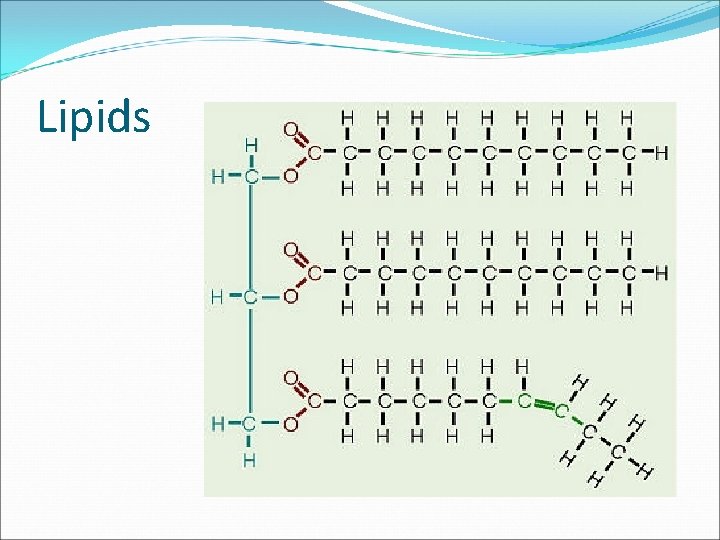
Lipids Saturated When all the carbon atoms in a fatty acid are joined by single bonds, and so the fatty acid contains the maximum number of hydrogen atoms Unsaturated Bent in shape so they don’t pack together so tightly-remain liquid at room temperature

Lipids

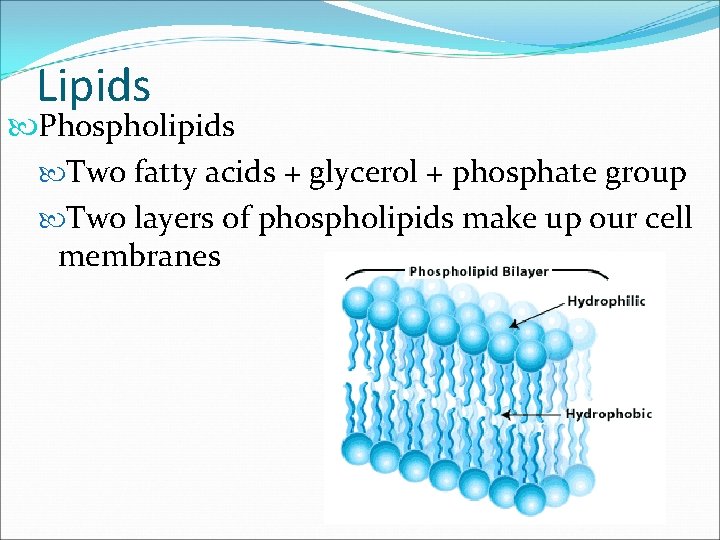
Lipids Phospholipids Two fatty acids + glycerol + phosphate group Two layers of phospholipids make up our cell membranes

Lipids Functions Insulation Energy Building cell membranes

Proteins Perform many, many, many functions throughout the body based on their unique shapes Elements C, H, O, N

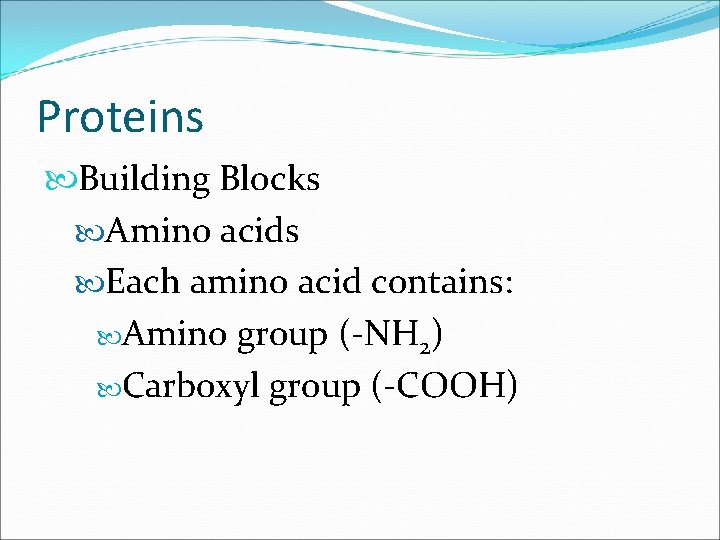
Proteins Building Blocks Amino acids Each amino acid contains: Amino group (-NH 2) Carboxyl group (-COOH)

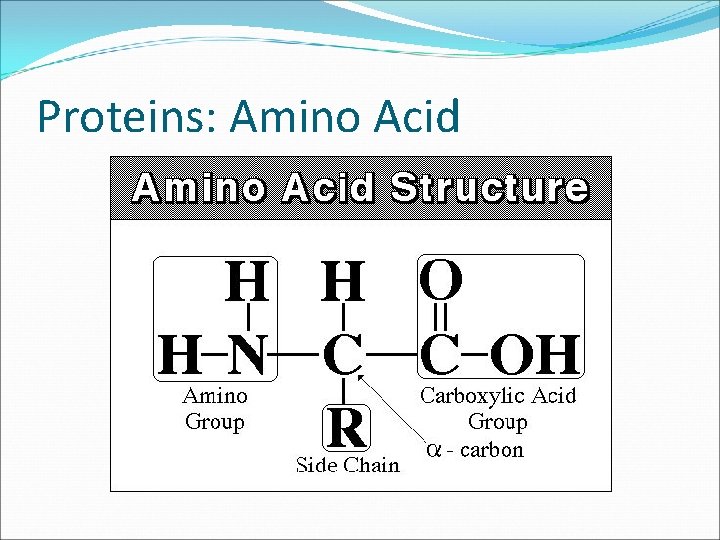
Proteins: Amino Acid

Proteins Polymers Polypeptides: strings of many amino acids Polypeptides have a unique 3 D structure that allows it to function

Proteins Functions Energy Building muscles Building enzymes (chemical reactions) Transporting materials Etc. , etc.

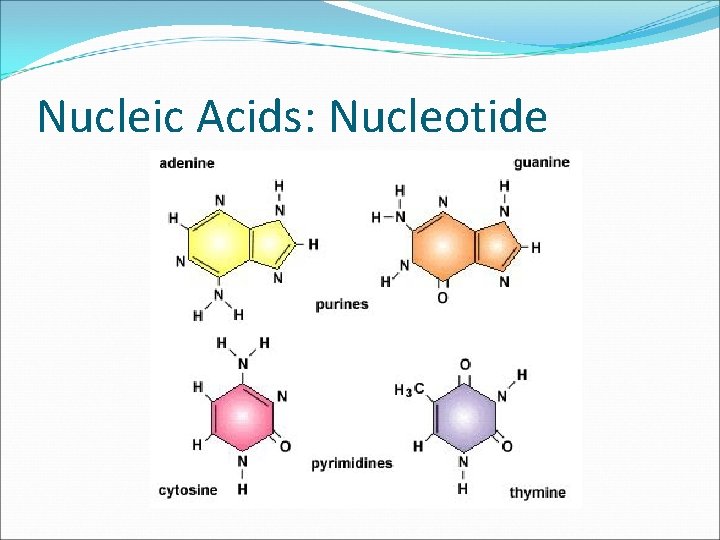
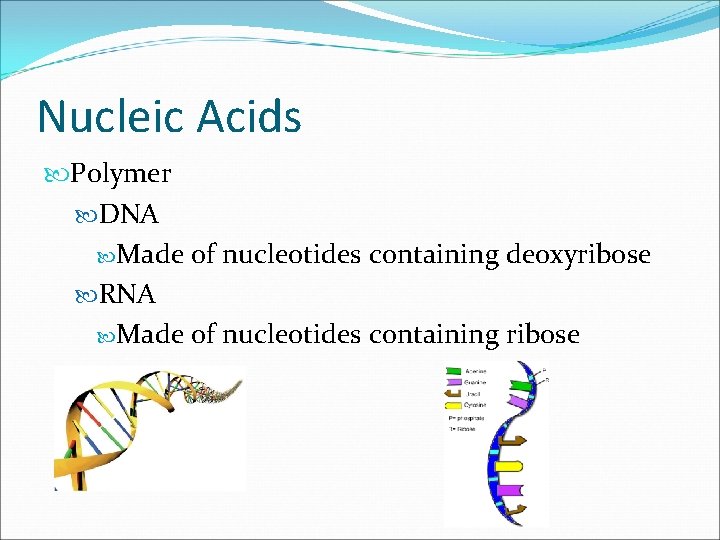
Nucleic Acids Compound Nucleic acids: polymer of nucleotides Elements C, H, O, N, P Building Blocks Nucleotides Consist of a 5 -carbon sugar, a phosphate group, and a nitrogenous base.

Nucleic Acids: Nucleotide

Nucleic Acids Polymer DNA Made of nucleotides containing deoxyribose RNA Made of nucleotides containing ribose

Nucleic Acids Functions Contains information for making proteins Store and transmit genetic information.