4 Major Macromolecules 3 Objectives 1 Describe the
4 Major Macromolecules 3. Objectives: 1. Describe the function of carbohydrates. 2. Draw the structure of a carbohydrate. Explain how carbohydrates are broken down and built.
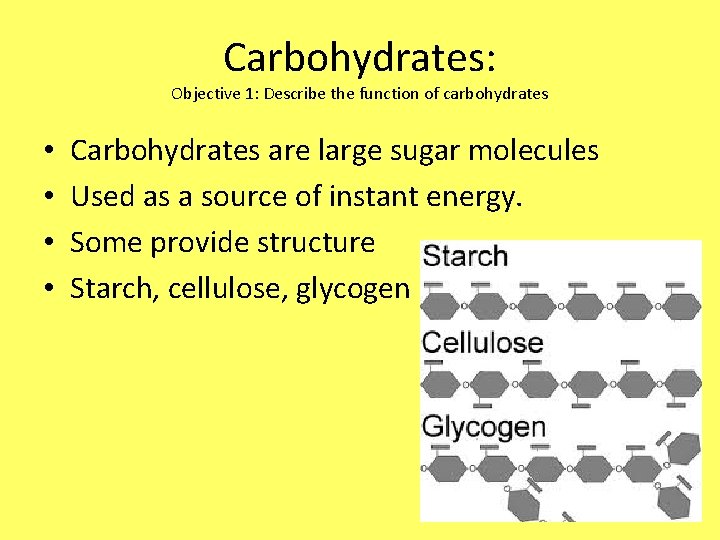
Carbohydrates: Objective 1: Describe the function of carbohydrates • • Carbohydrates are large sugar molecules Used as a source of instant energy. Some provide structure Starch, cellulose, glycogen

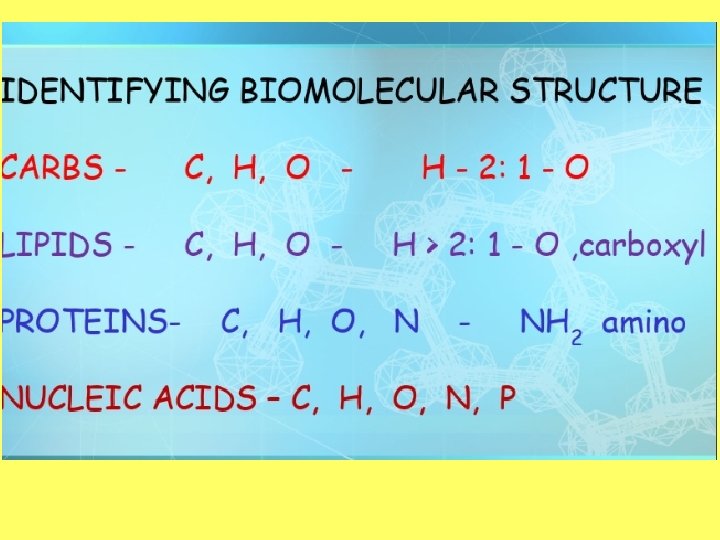
Structure of a carbohydrate: Objective 2: Describe the structure of a carbohydrate • Monomer—monosaccharide (C 6 H 12 O 6) • 2 monosaccharides=disaccharide (C 12 H 22 O 11) • 3 or more monosaccharides=polysaccharide Hydrogen is always in a 2: 1 ratio with oxygen!


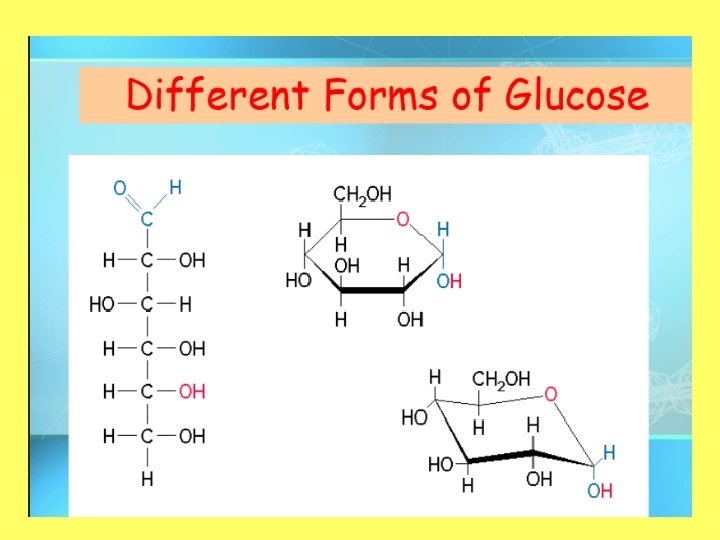
Three Monosaccharides • All isomers of each other • Examples: glucose, fructose, galactose, deoxyribose, ribose
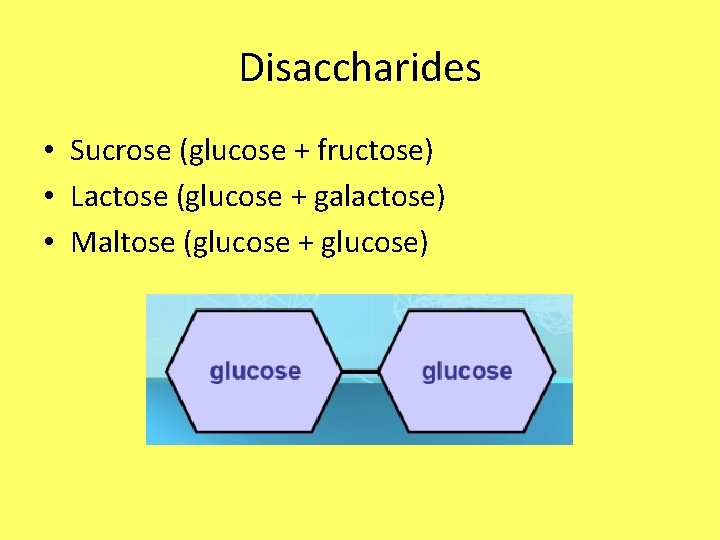
Disaccharides • Sucrose (glucose + fructose) • Lactose (glucose + galactose) • Maltose (glucose + glucose)
Carbohydrate models • Show the dehydration synthesis reaction for the formation of a sucrose molecule. Label each molecule in the reaction. • Show the hydrolysis of maltose. Label each molecule in the reaction.
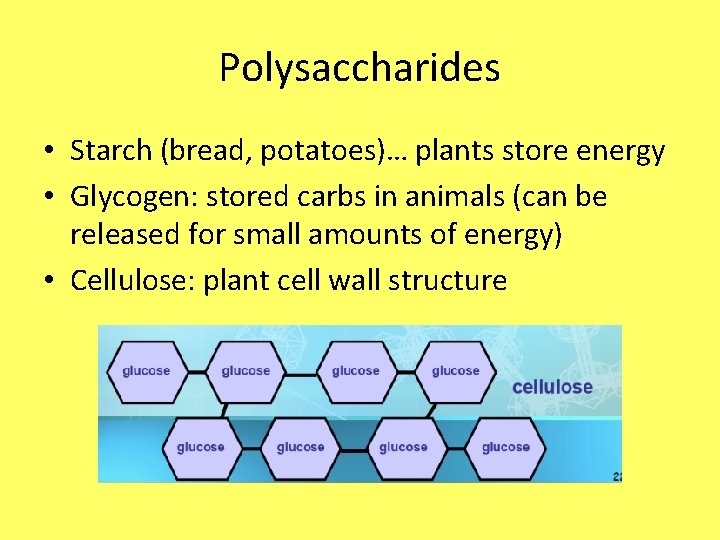
Polysaccharides • Starch (bread, potatoes)… plants store energy • Glycogen: stored carbs in animals (can be released for small amounts of energy) • Cellulose: plant cell wall structure
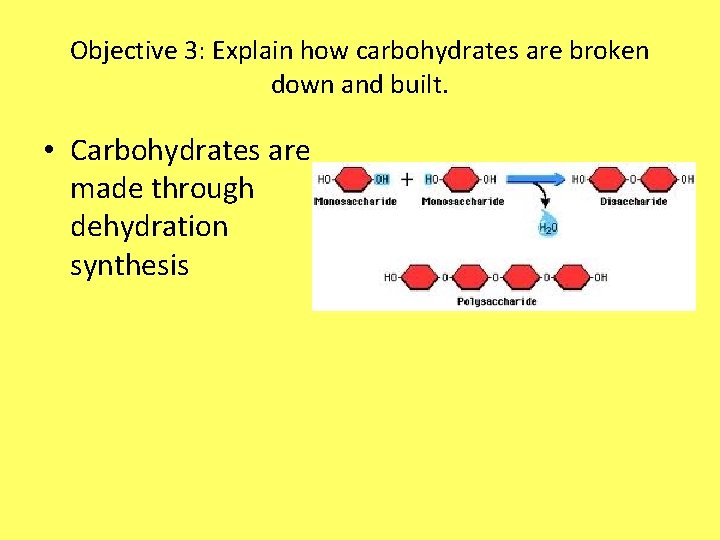
Objective 3: Explain how carbohydrates are broken down and built. • Carbohydrates are made through dehydration synthesis
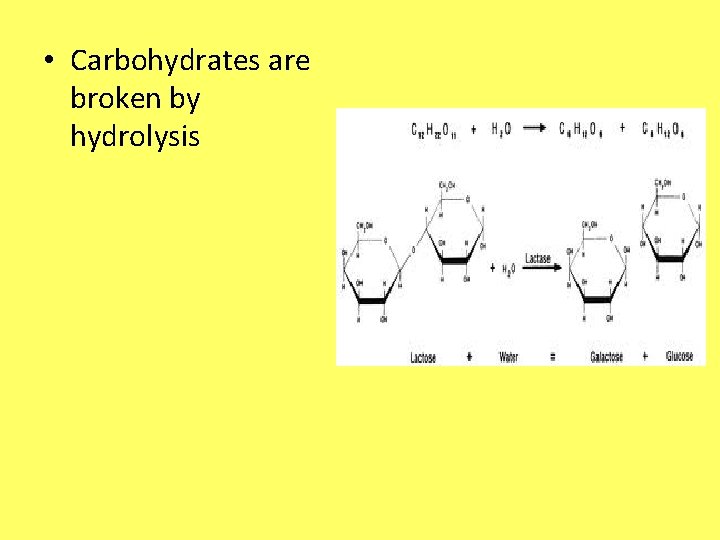
• Carbohydrates are broken by hydrolysis

Chemical Tests for Organic Lab • Benedict’s solution—changes color from blue to yellow/orange when heated to indicate simple sugars (monosaccharides) • Lugol’s Iodine—changes color from yellow to black/purple to indicate polysaccharides (starch, cellulose, etc. )

Review of Objectives • Objectives: 1. Describe the function of carbohydrates. 2. Draw the structure of a carbohydrate. 3. Explain how carbohydrates are broken down and built.

Lipids Objectives: 1. Describe the function of lipids 2. Draw and label the structure of a lipid 3. Differentiate among the different forms of lipids

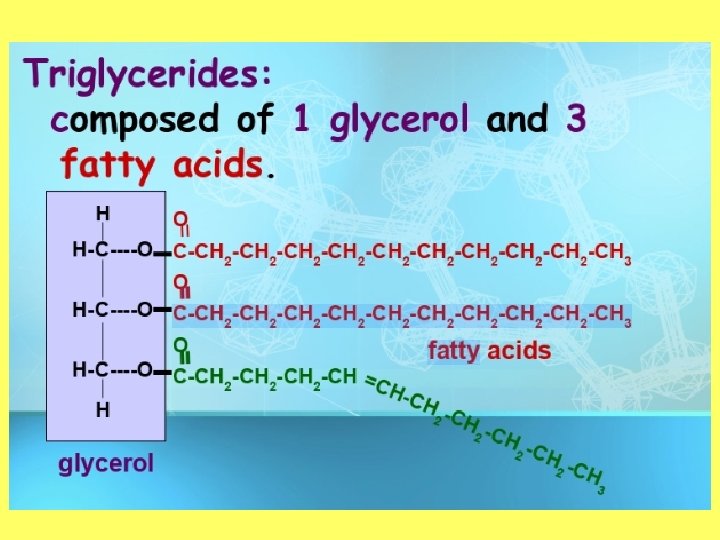
Objective 1: Describe the function of lipids • Compounds that are not soluble in water • Examples of lipids: – Fats (necessary to living tissues) – phospholipids – Oils – Wax – Steroid hormones – triglycerides
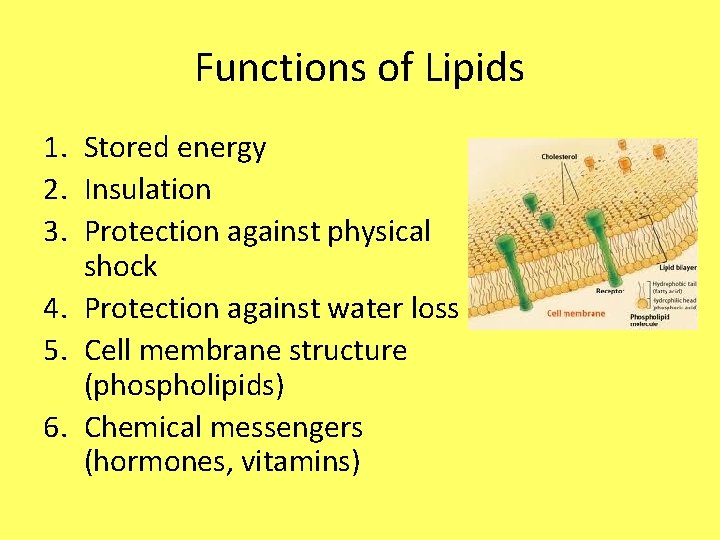
Functions of Lipids 1. Stored energy 2. Insulation 3. Protection against physical shock 4. Protection against water loss 5. Cell membrane structure (phospholipids) 6. Chemical messengers (hormones, vitamins)

Objective 2: draw and label the structure of a lipid Structure of a Lipid • Polymer: Lipid (triglyceride) • Monomers: Fatty Acid and glycerol • Made up of carbon, hydrogen, and oxygen (carboxyl) • Greater than 2: 1 ratio of H: O
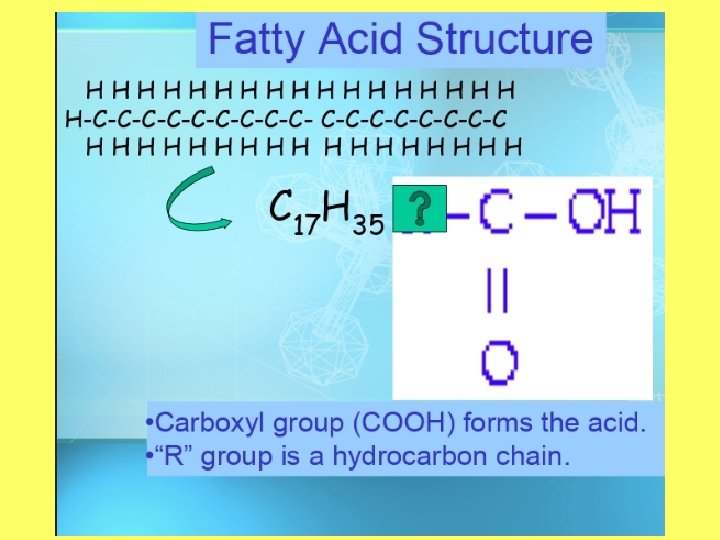
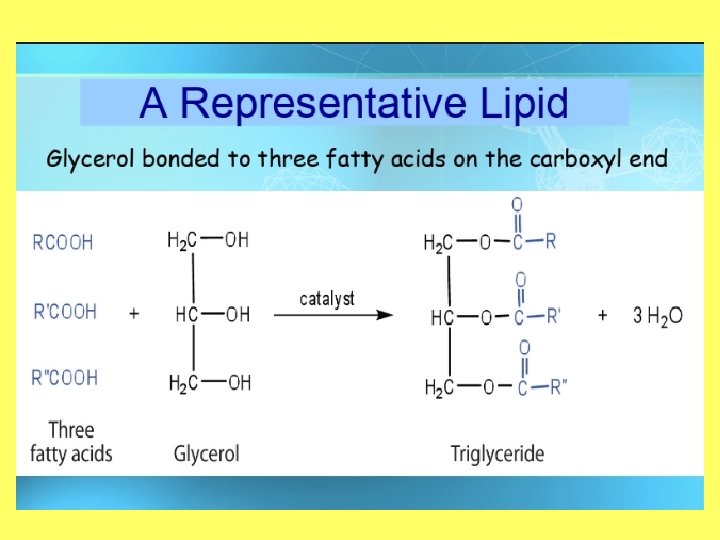
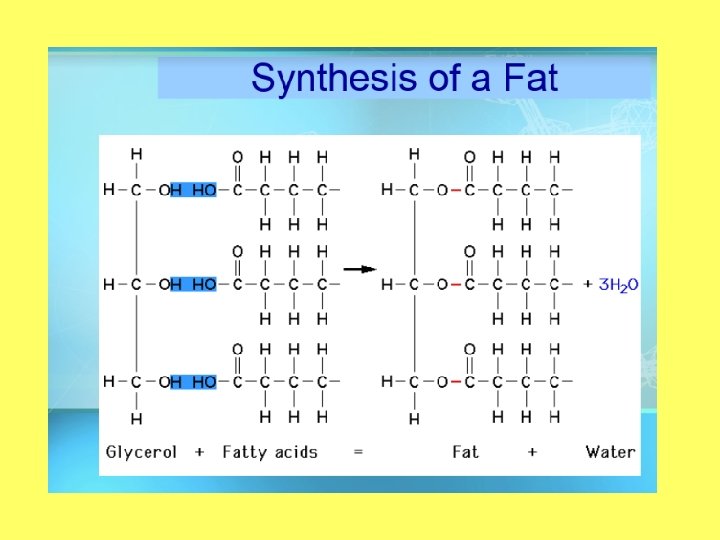

Carbs vs. Lipids

2 Main Types of Fatty Acid Tails • Saturated – No double bonds – Maximum number of hydrogens – Not healthy – Solid fats at room temperature • Unsaturated – At least one C=C (carbon, carbon double bond) – Low number of hydrogens – Oils – Better for you (olives, avocados, nuts) – Looks “bent”
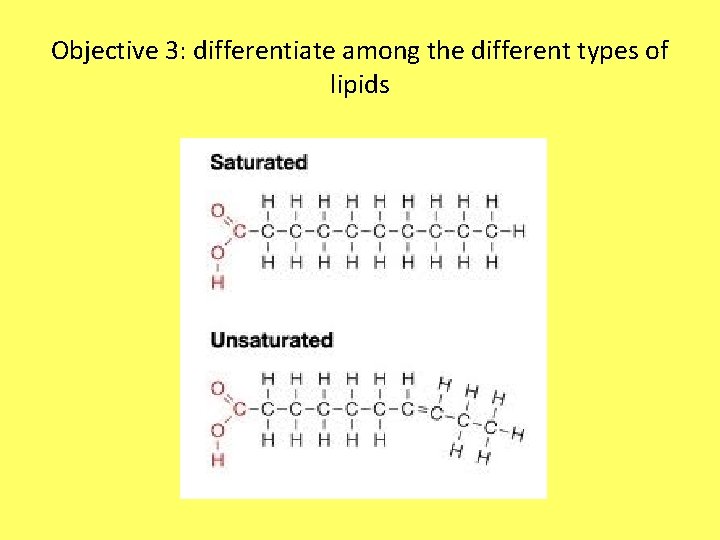
Objective 3: differentiate among the different types of lipids

Chemical Tests • Brown paper bag test – Rub sample of organic substance on a brown paper bag. Lipids will leave a translucent spot (grease spot) when dry. • Sudan IV—turns red to indicate lipids

Review of Objectives • Objective 1: describe the function of lipids • Objective 2: draw and label a lipid • Objective 3: differentiate among the different types of lipids

Nucleic Acid Objectives: 1. Describe the function of nucleic acids 2. Draw and label the structure of a nucleotide 3. Explain how nucleotides link together to make nucleic acids
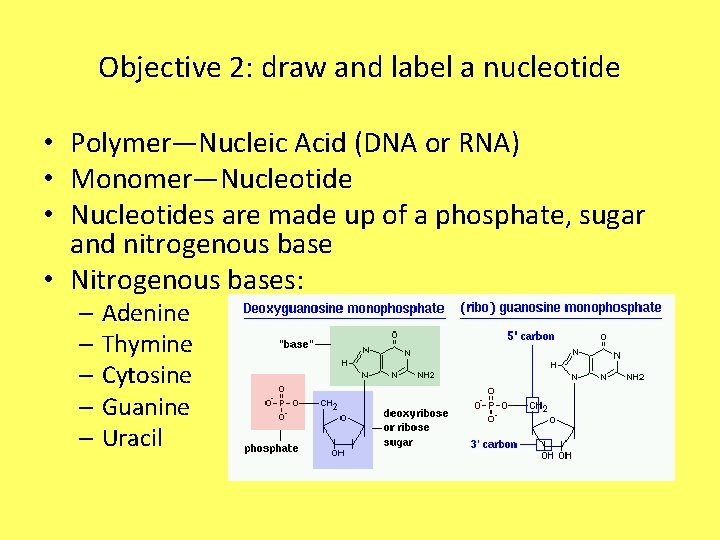
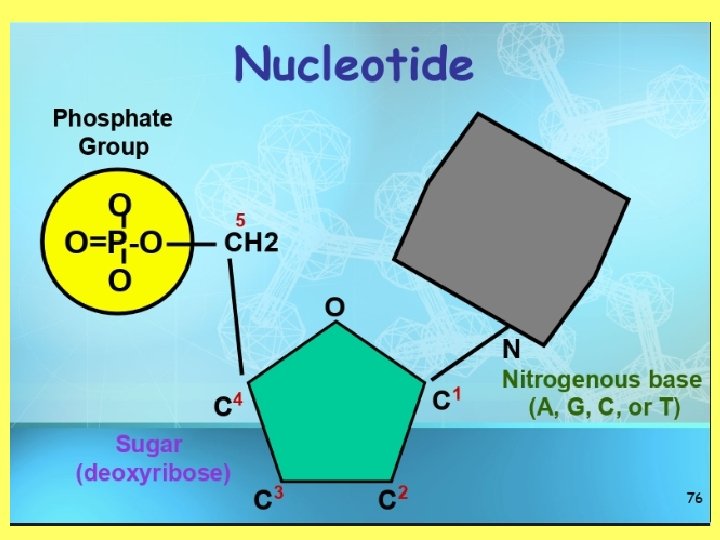
Objective 2: draw and label a nucleotide • Polymer—Nucleic Acid (DNA or RNA) • Monomer—Nucleotide • Nucleotides are made up of a phosphate, sugar and nitrogenous base • Nitrogenous bases: – Adenine – Thymine – Cytosine – Guanine – Uracil
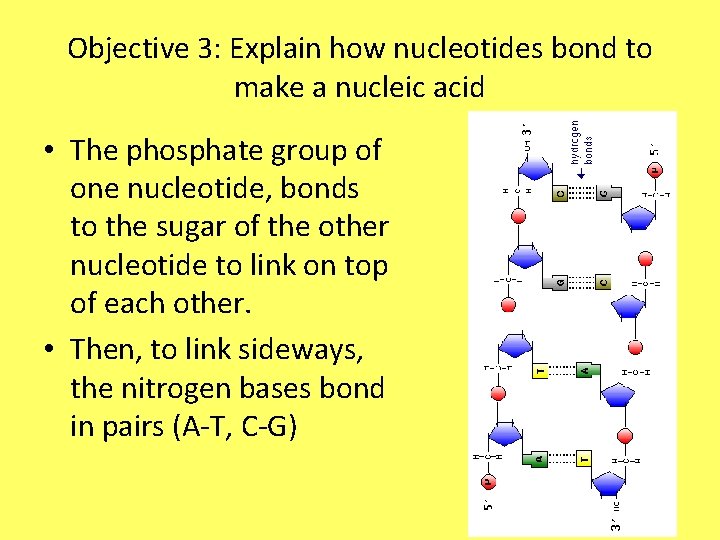
Objective 3: Explain how nucleotides bond to make a nucleic acid • The phosphate group of one nucleotide, bonds to the sugar of the other nucleotide to link on top of each other. • Then, to link sideways, the nitrogen bases bond in pairs (A-T, C-G)
2 Types of Nucleic Acids • DNA – Codes for proteins – Sugar—deoxyribose (“without oxygen”) – Double helical shape – Bases: adenine, thymine, cytosine, guanine • RNA – Codes for proteins – Sugar—ribose – One stranded—no double helix – Bases: adenine, uracil, cytosine, guanine
Proteins Objectives: 1. Explain the function of proteins 2. Explain how amino acids link to make proteins
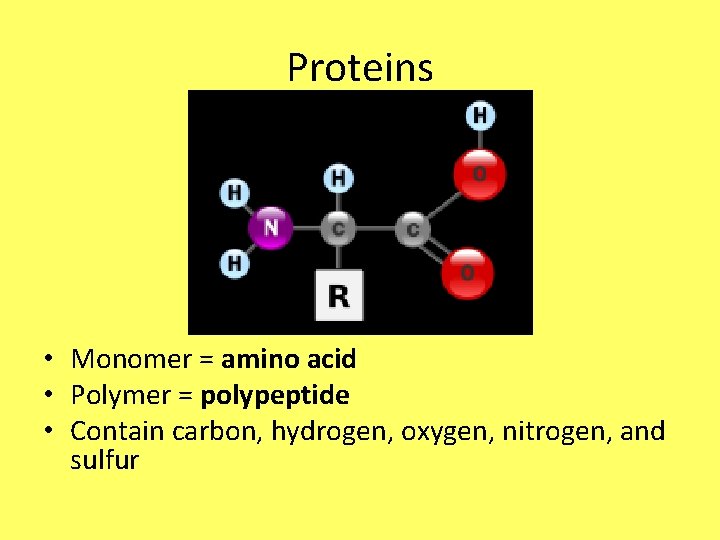
Proteins • Monomer = amino acid • Polymer = polypeptide • Contain carbon, hydrogen, oxygen, nitrogen, and sulfur
Function • Amino acids bond together by peptide bonds to form polypeptides • Six functions of proteins: 1. 2. 3. 4. 5. 6. Storage (albumin—egg white) Transport (hemoglobin) Regulatory (hormones) Movement (muscles) Structural (membranes, hair, nails) Enzymes (cellular reactions)
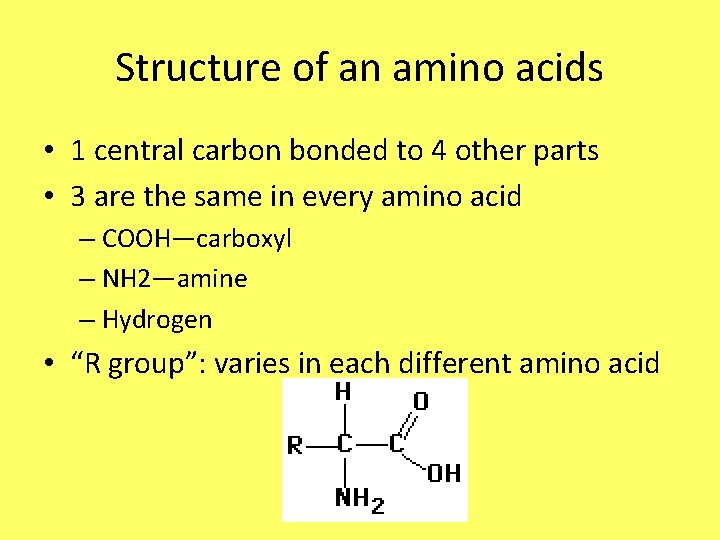
Structure of an amino acids • 1 central carbon bonded to 4 other parts • 3 are the same in every amino acid – COOH—carboxyl – NH 2—amine – Hydrogen • “R group”: varies in each different amino acid
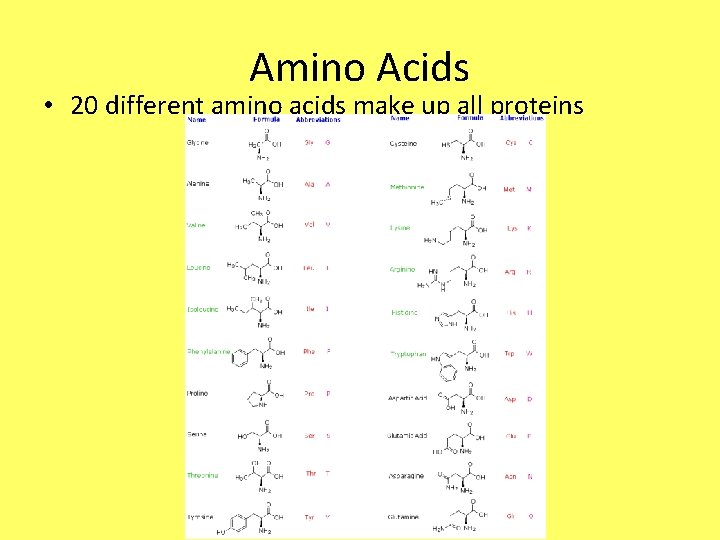
Amino Acids • 20 different amino acids make up all proteins
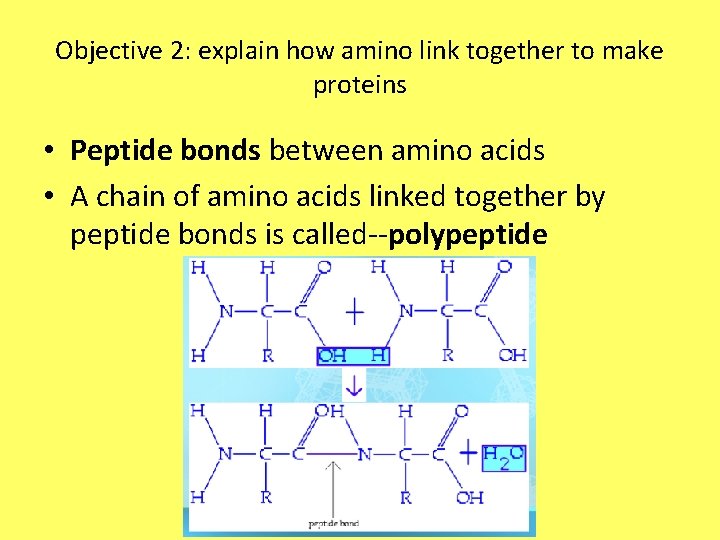
Objective 2: explain how amino link together to make proteins • Peptide bonds between amino acids • A chain of amino acids linked together by peptide bonds is called--polypeptide
Protein Structure • Polypeptides are put together and fold in specific shapes to make different protein molecules • In proteins– structure = function

Chemical Tests • Biuret solution—changes color from blue to lavender/purple to indicate proteins

Enzymes

Enzyme Questions 1. What does a catalyst do? 2. What is an enzyme? 3. Why do enzymes generally bind to only one type of substrate? 4. How are the “lock and key” and “induced fit” models similar? 5. How are the “lock and key” and “induced fit” models different? 6. What are 3 factors that can affect the way enzymes work? Explain how each factor would affect an enzyme.
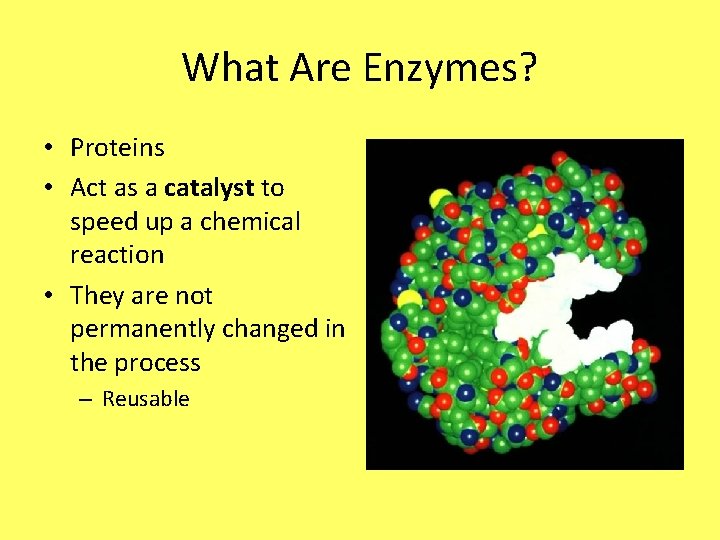
What Are Enzymes? • Proteins • Act as a catalyst to speed up a chemical reaction • They are not permanently changed in the process – Reusable
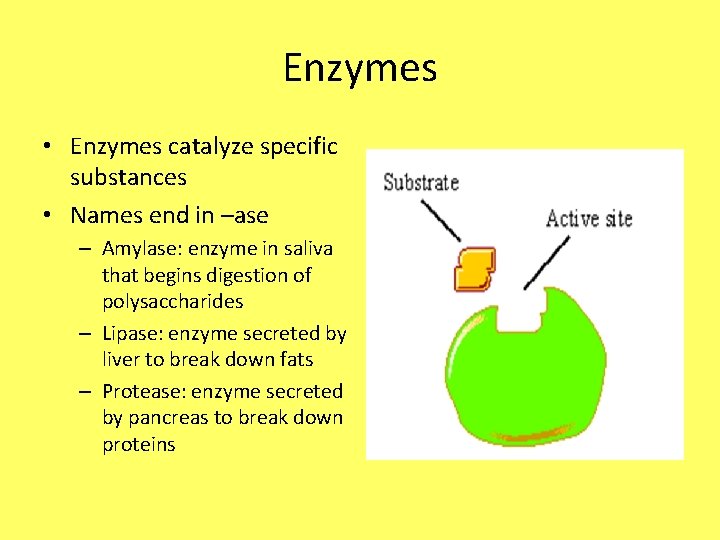
Enzymes • Enzymes catalyze specific substances • Names end in –ase – Amylase: enzyme in saliva that begins digestion of polysaccharides – Lipase: enzyme secreted by liver to break down fats – Protease: enzyme secreted by pancreas to break down proteins
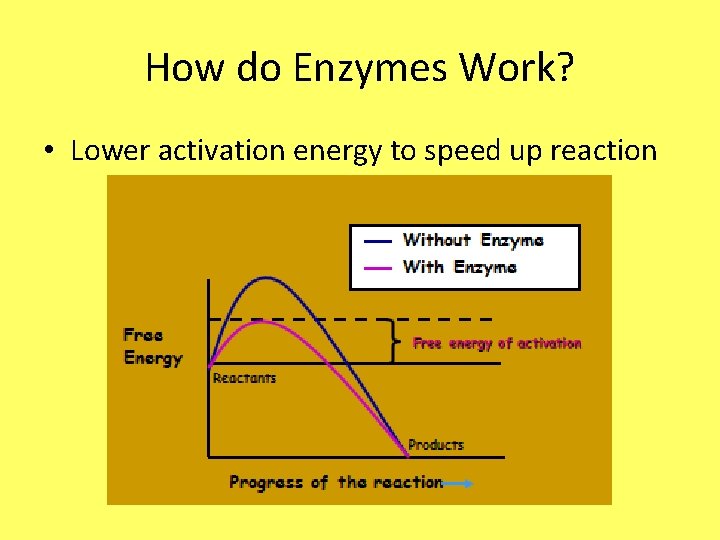
How do Enzymes Work? • Lower activation energy to speed up reaction
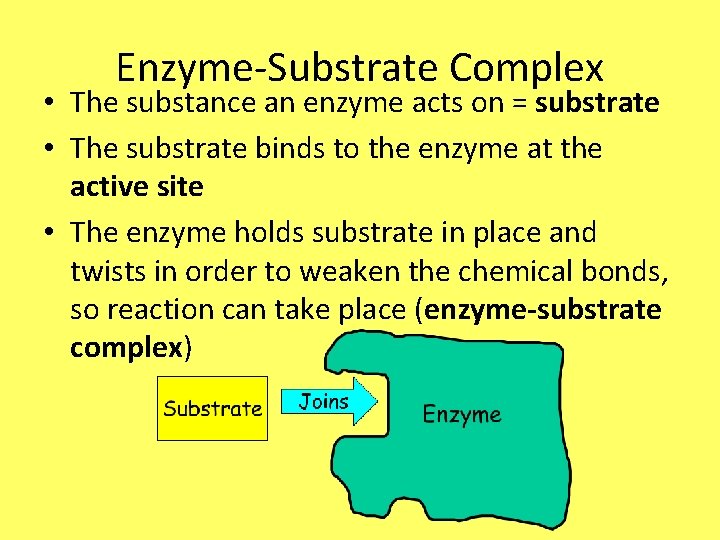
Enzyme-Substrate Complex • The substance an enzyme acts on = substrate • The substrate binds to the enzyme at the active site • The enzyme holds substrate in place and twists in order to weaken the chemical bonds, so reaction can take place (enzyme-substrate complex)
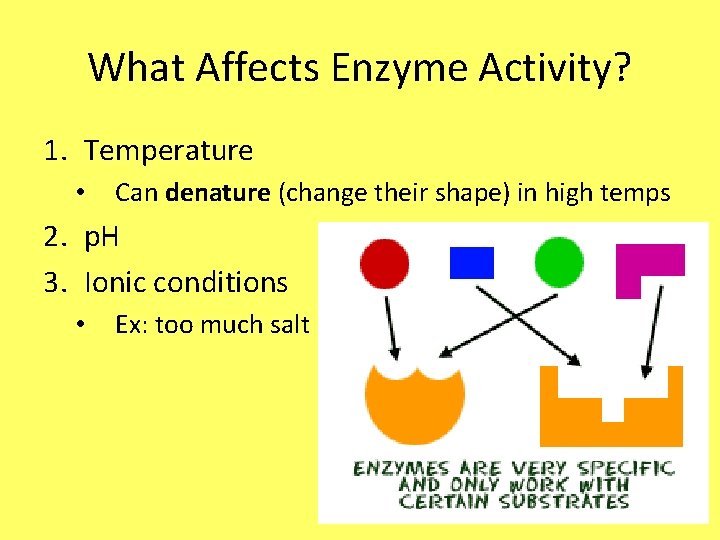
What Affects Enzyme Activity? 1. Temperature • Can denature (change their shape) in high temps 2. p. H 3. Ionic conditions • Ex: too much salt
- Slides: 47