4 B Section 2 The Structure of Atoms

4 B = Section 2 The Structure of Atoms p 119 -127 (Question 14 -17 will be on 4 B quiz, too. ) Physical Science 08 -09 Curriculum 6/9/2021 Ph. Sci Ch 4 17 Questions 1

Give the relative masses of protons, neutrons & electrons n n Protons & neutrons have about the same mass; Electrons are over 1, 000 times smaller (in mass than protons & neutrons). 6/9/2021 http: //en. wikipedia. org/wiki/File: Atomic_resolution_Au 100. JPG Ph. Sci Ch 4 17 Questions 19 slides 2
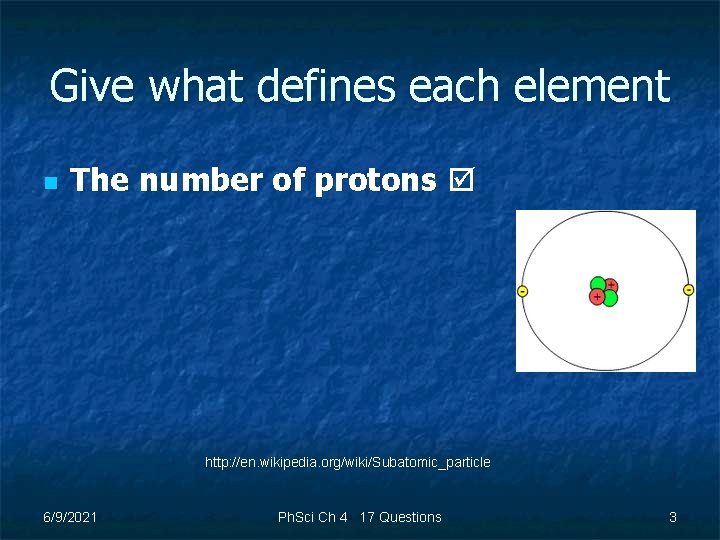
Give what defines each element n The number of protons http: //en. wikipedia. org/wiki/Subatomic_particle 6/9/2021 Ph. Sci Ch 4 17 Questions 3

Explain why most (unreacted) atoms do not have an overall charge if they are made of charged particles n n n Most atoms have an equal number of protons and electrons whose charges exactly cancel. 6/9/2021 Ph. Sci Ch 4 17 Questions 4

Define ion n an atom who has gained or lost an electron and therefore ( ) is charged. 6/9/2021 Ph. Sci Ch 4 17 Questions 5
Explain what holds atoms together n n n Attractions between the opposite charges of protons and electrons 6/9/2021 Ph. Sci Ch 4 17 Questions 6
Explain how different elements have their own unique properties n n n The atoms of each type of element have their own different structure 6/9/2021 Ph. Sci Ch 4 17 Questions 7

Define atomic number n how many protons are in an atom 6/9/2021 Ph. Sci Ch 4 17 Questions 8

Give the largest naturally occurring element and its atomic number n n Uranium, 92 6/9/2021 Ph. Sci Ch 4 17 Questions 9

Define mass number n n n Tells the number of protons plus the number of neutrons in an atom. 6/9/2021 Ph. Sci Ch 4 17 Questions 10
Define isotope n n n An atom that has the same number of protons but a different number of neutrons. 6/9/2021 Ph. Sci Ch 4 17 Questions 11

Define radioisotopes n n Unstable isotopes which emits radiation and decays into stable forms (this might take a long time and many steps). 6/9/2021 Ph. Sci Ch 4 17 Questions 12
Define unified atomic mass unit n n n A quantity used to measure the mass of atoms (which is equal to 1/12 of a carbon 12 atom) And is abbreviated u. (Sometimes called an amu) 6/9/2021 Ph. Sci Ch 4 17 Questions 13

Define average atomic mass n n n average of the mass of an element’s atoms found in nature where common isotopes are more important 6/9/2021 Ph. Sci Ch 4 17 Questions 14
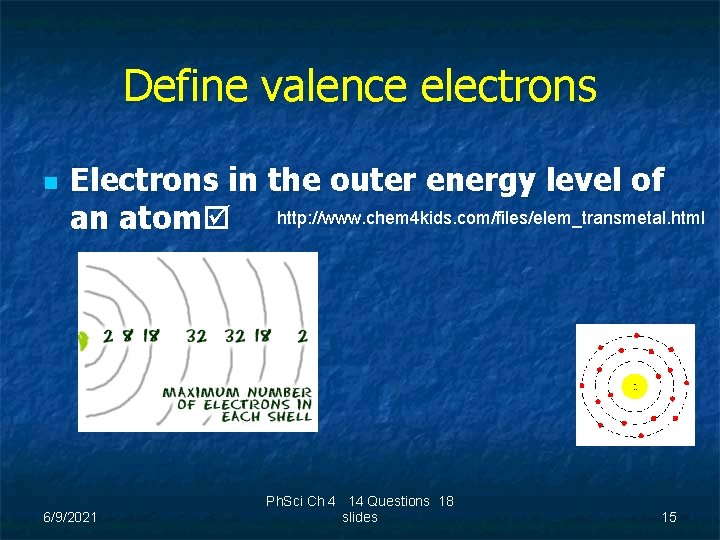
Define valence electrons n Electrons in the outer energy level of an atom http: //www. chem 4 kids. com/files/elem_transmetal. html 6/9/2021 Ph. Sci Ch 4 14 Questions 18 slides 15

*** Using a periodic table, give the # of protons, neutrons, & electrons of various atoms 6/9/2021 Ph. Sci Ch 4 17 Questions 16

*** Calculate atomic number & mass number 6/9/2021 Ph. Sci Ch 4 17 Questions 17

*** Write isotopes using the forms given on p 124 (Fig 5 and in the reading) 6/9/2021 Ph. Sci Ch 4 17 Questions 18

n The end 6/9/2021 Ph. Sci Ch 4 17 Questions 19
- Slides: 19