4 3 Types of Chemical Reactions Synthesis Synthesis




- Slides: 13

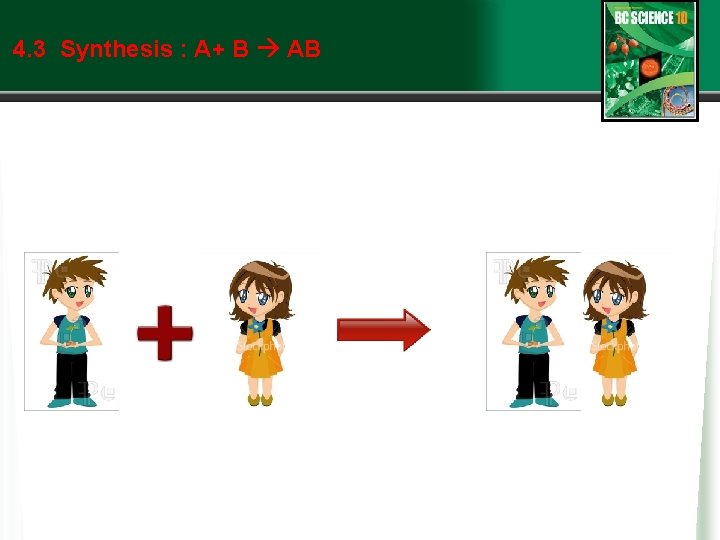
4. 3 Types of Chemical Reactions: Synthesis • Synthesis reactions are also known as formation reactions. w Two or more reactants (usually elements) join to form a compound. w A + B AB where A and B represent elements w The elements may form ionic compounds: w Sodium metal and chlorine gas combine to form sodium chloride. w 2 Na + Cl 2 2 Na. Cl w Magnesium metal reacts with oxygen gas to form magnesium oxide. w 2 Mg + O 2 2 Mg. O w Or the elements may form covalent compounds, like this: See pages 258 - 259 (c) Mc. Graw Hill Ryerson 2007

4. 3 Synthesis : A+ B AB

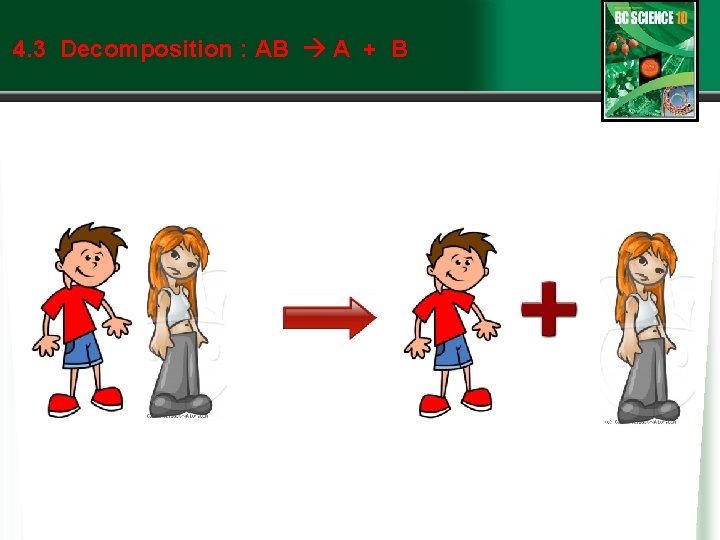
4. 3 Types of Chemical Reactions: Decomposition • Decomposition reactions are the opposite of synthesis reactions. w A compounds breaks down into two or more products (often elements). w AB A + B where A and B represent elements w Ionic compounds may decompose to produce elements, like this: w Table salt, sodium chloride, can be broken down into sodium metal and chlorine gas by melting salt at 800ºC and running electricity through it. w 2 Na. Cl 2 Na + Cl 2 w Or covalent compounds may decompose into elements, like this: w By running electricity through water, the See page 260 (c) Mc. Graw Hill Ryerson 2007

4. 3 Decomposition : AB A + B

4. 3 Types of Chemical Reactions: Single Replacement • Single replacement reactions replace one element from a compound with a separate element added as a reactant. w A compound an element reactant, and the element switches places with part of the original compound. § A + BC B + AC where A is a metal, or § A + BC C + BA where A is a non-metal w When A is a metal: w Cu + Ag. NO 3 Ag + Cu(NO 3)2 w When A is a non-metal: w When fluorine is bubbled through a sodium iodide solution, iodine and sodium fluoride are produced. w F 2 + Na. I I 2 + Na. F See page 261 (c) Mc. Graw Hill Ryerson 2007

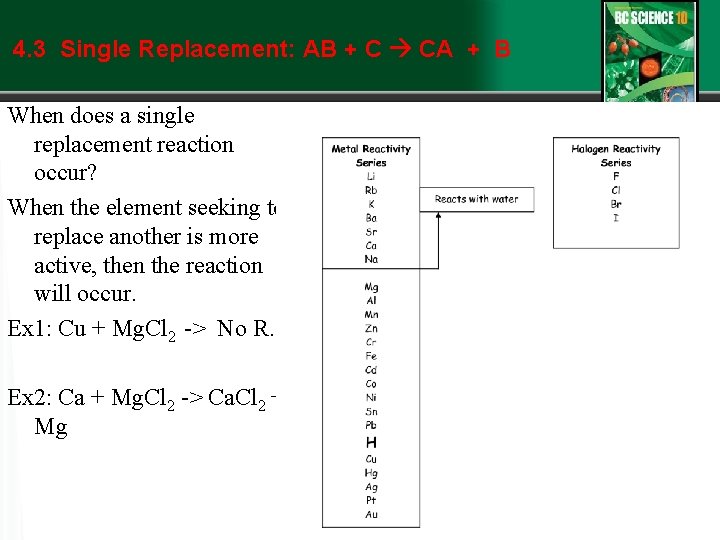
4. 3 Single Replacement: AB + C CA + B

4. 3 Single Replacement: AB + C CA + B When does a single replacement reaction occur? When the element seeking to replace another is more active, then the reaction will occur. Ex 1: Cu + Mg. Cl 2 -> No R. Ex 2: Ca + Mg. Cl 2 -> Ca. Cl 2 + Mg

4. 3 Types of Chemical Reactions: Double Replacement • Double replacement reactions swap elements between two compounds reacting together to form two new compounds. w Two compounds reactant, with elements switching places between the original compounds. w Two solutions react to form a precipitate (ppt, solid) and another solution § Ionic soln + ionic soln/ppt § AB + CD AD + BC w When potassium chromate and silver nitrate react, they form a red precipitate, silver chromate, in a solution of potassium nitrate. w K 2 Cr. O 4 + Ag. NO 3 Ag 2 Cr. O 4 + KNO 3 See page 262 (c) Mc. Graw Hill Ryerson 2007

4. 3 Double Replacement: AB + CD CA + BD

4. 3 Double Replacement: AB + CD CA + BD Double Replacement reactions will occur if any one of these happen: 1. Water is formed 2. A gas is formed 3. A precipitate or solid is formed (Check a solubility table to predict this)

4. 3 Types of Chemical Reactions: Neutralization (aka Acid-Base reactions) • Neutralization reactions occur when an acid and a base react to form a salt and water. w An acid (most compounds starting with H) and a base (most compounds ending in OH, or beginning with NH 4) react. § Acid + base salt + water § HX + MOH MX (a salt) + HOH (= H 2 O) where X and M are elements w Sulphuric acid is used to neutralize calcium hydroxide: w H 2 SO 4 + Ca(OH) 2 Ca. SO 4 + 2 H 2 O w Phosphoric acid helps to neutralize the compounds that cause rust, such as iron (II) hydroxide. w H 3 PO 4 + 3 Fe(OH)2 Fe 3(PO 4)2 + 6 H 2 O See page 263 (c) Mc. Graw Hill Ryerson 2007

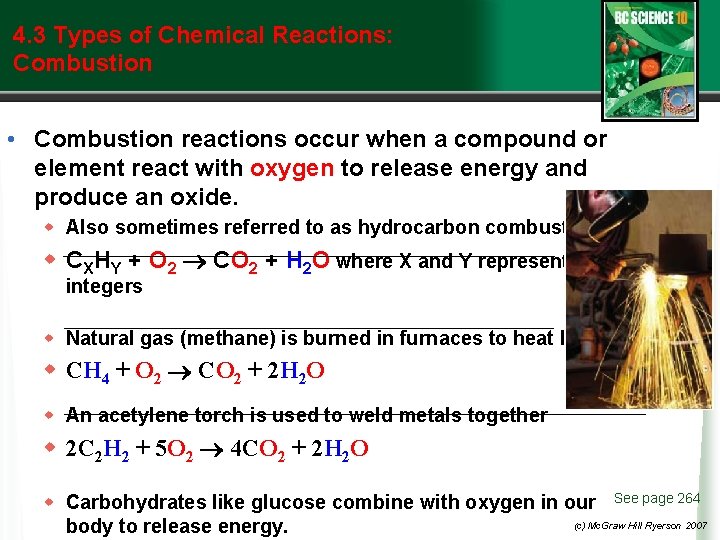
4. 3 Types of Chemical Reactions: Combustion • Combustion reactions occur when a compound or element react with oxygen to release energy and produce an oxide. w Also sometimes referred to as hydrocarbon combustion w CXHY + O 2 CO 2 + H 2 O where X and Y represent integers w Natural gas (methane) is burned in furnaces to heat homes. w CH 4 + O 2 CO 2 + 2 H 2 O w An acetylene torch is used to weld metals together w 2 C 2 H 2 + 5 O 2 4 CO 2 + 2 H 2 O w Carbohydrates like glucose combine with oxygen in our See page 264 (c) Mc. Graw Hill Ryerson 2007 body to release energy.

4. 3 Types of Chemical Reactions: Summary of Types See page 265 Take the Section 6. 1 Quiz (c) Mc. Graw Hill Ryerson 2007