4 3 APPLICATION EXAMPLE FOR MOLES Gas Behavior

4. 3 APPLICATION EXAMPLE FOR MOLES Gas Behavior How does the behavior of gases affect airbags?

What is pressure? Pressure – Force of gas particles running into a surface
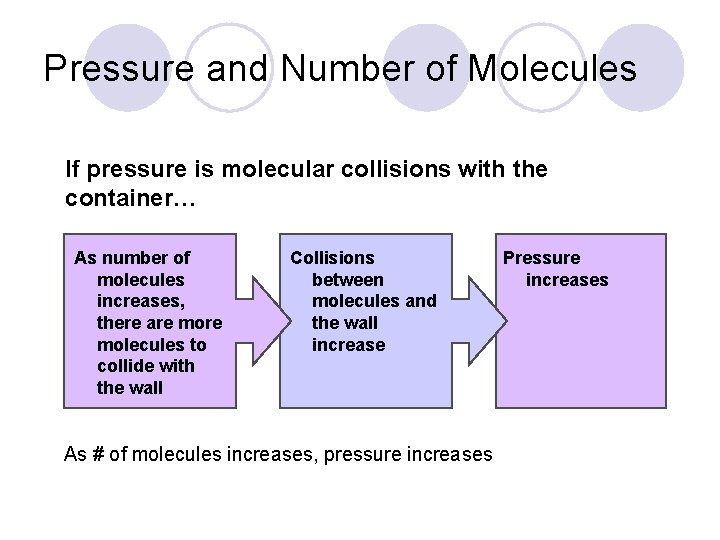
Pressure and Number of Molecules If pressure is molecular collisions with the container… As number of molecules increases, there are molecules to collide with the wall Collisions between molecules and the wall increase As # of molecules increases, pressure increases Pressure increases
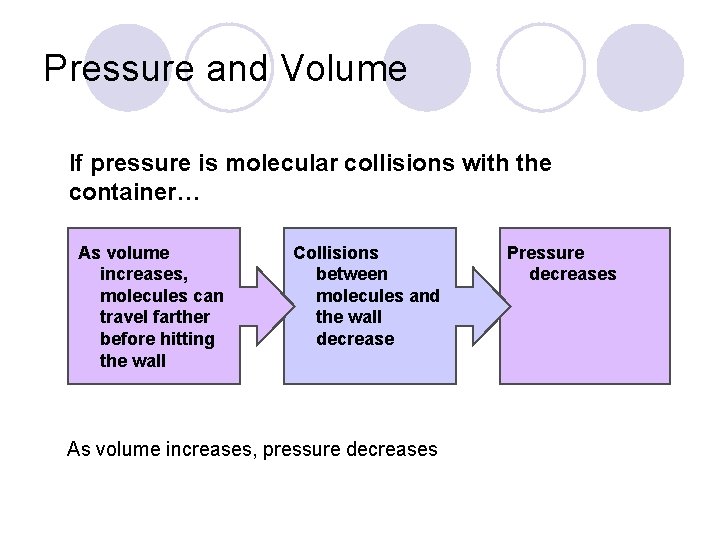
Pressure and Volume If pressure is molecular collisions with the container… As volume increases, molecules can travel farther before hitting the wall Collisions between molecules and the wall decrease As volume increases, pressure decreases Pressure decreases
Section 4. 3—Counting Molecules So the number of molecules affects pressure of an airbag…how do we “count” molecules?

l How do auto makers utilize enough atoms of gas to create pressure that inflates the bag, l But not so many that the pressure is too great when it hits you and your face that it causes permanent damage l Count the atoms

What is a mole?

Definition Mole – SI unit for counting The only acceptable abbreviation for “mole” is “mol”…not “m”!!

What is a counting unit? You’re already familiar with one counting unit…a “dozen” A dozen = 12 “Dozen” 12 A dozen doughnuts 12 doughnuts A dozen books 12 books A dozen cars 12 cars A dozen people 12 people

What does a “mole” count in? A mole = 6. 02 1023 (called Avogadro’s number) 6. 02 1023 = 602, 000, 000, 000 “mole” 6. 02 1023 1 mole of doughnuts 6. 02 1023 doughnuts 1 mole of atoms 6. 02 1023 atoms 1 mole of molecules 6. 02 1023 molecules This means a 12 ounce bottle of water would have 19. 7 “moles” of water…a much easier-to-work-with number!

Molar Mass
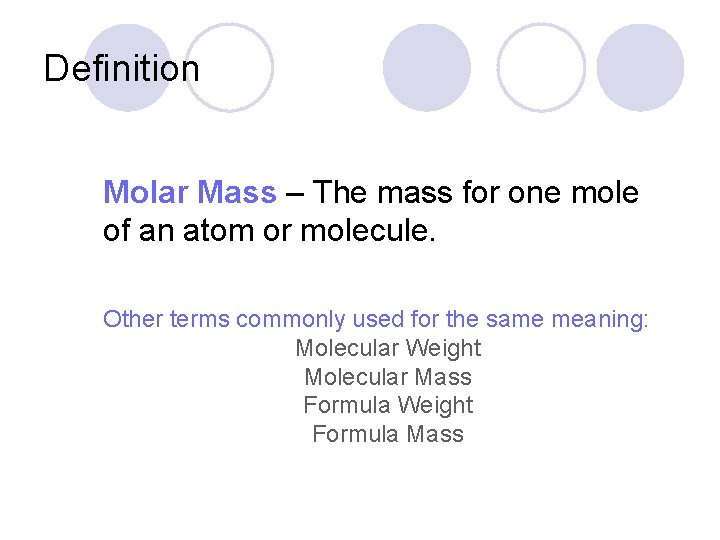
Definition Molar Mass – The mass for one mole of an atom or molecule. Other terms commonly used for the same meaning: Molecular Weight Molecular Mass Formula Weight Formula Mass
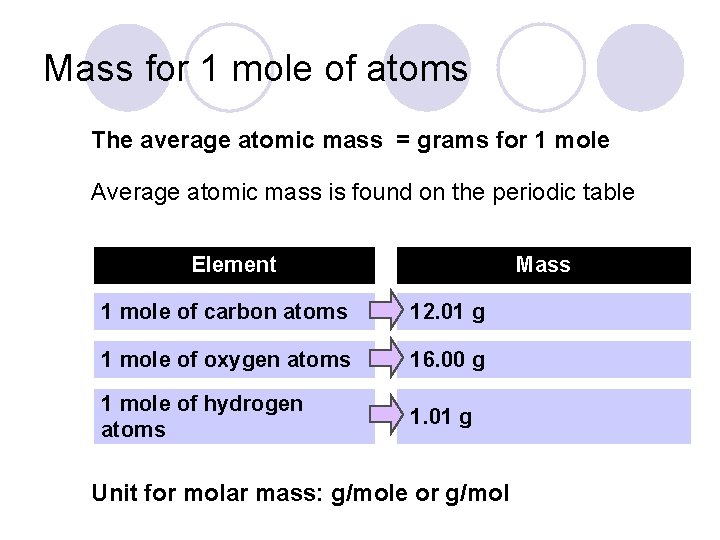
Mass for 1 mole of atoms The average atomic mass = grams for 1 mole Average atomic mass is found on the periodic table Element Mass 1 mole of carbon atoms 12. 01 g 1 mole of oxygen atoms 16. 00 g 1 mole of hydrogen atoms 1. 01 g Unit for molar mass: g/mole or g/mol

Molar mass for molecules The molar mass for a molecule = the sum of the molar masses of all the atoms
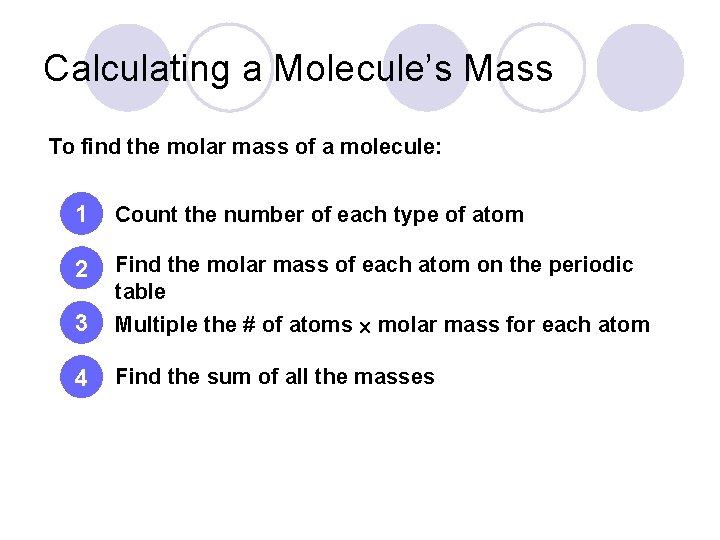
Calculating a Molecule’s Mass To find the molar mass of a molecule: 1 Count the number of each type of atom 2 Find the molar mass of each atom on the periodic table 3 Multiple the # of atoms molar mass for each atom 4 Find the sum of all the masses
Example: Molar Mass Example: Find the molar mass for Ca. Br 2
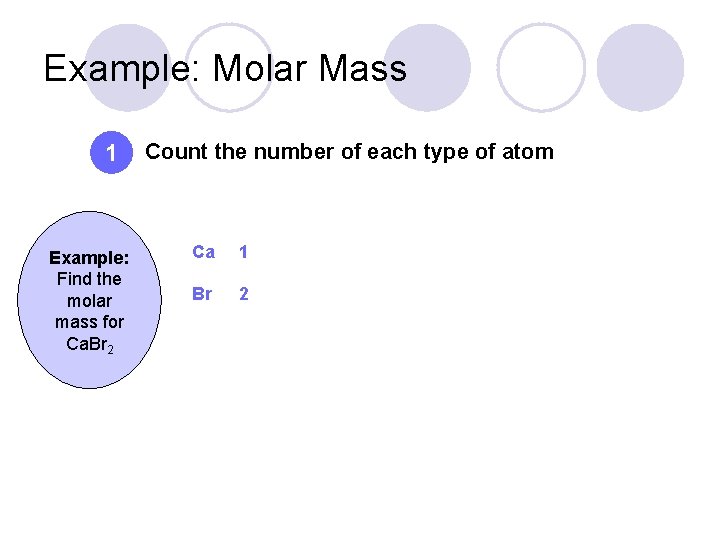
Example: Molar Mass 1 Example: Find the molar mass for Ca. Br 2 Count the number of each type of atom Ca 1 Br 2
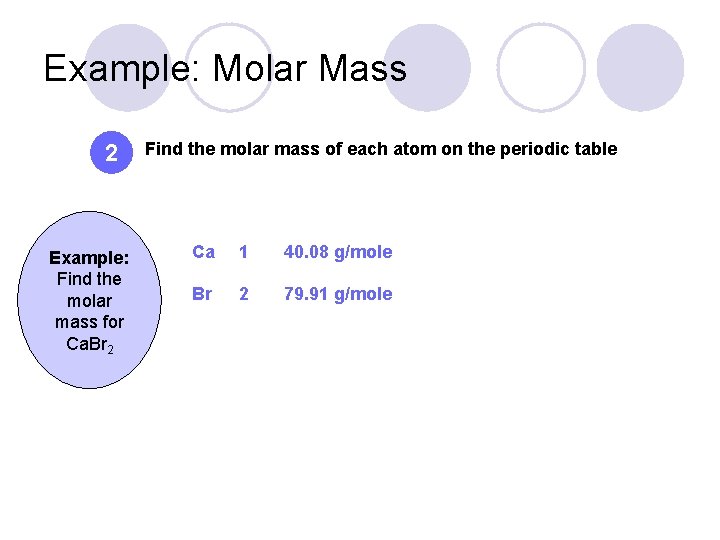
Example: Molar Mass 2 Example: Find the molar mass for Ca. Br 2 Find the molar mass of each atom on the periodic table Ca 1 40. 08 g/mole Br 2 79. 91 g/mole
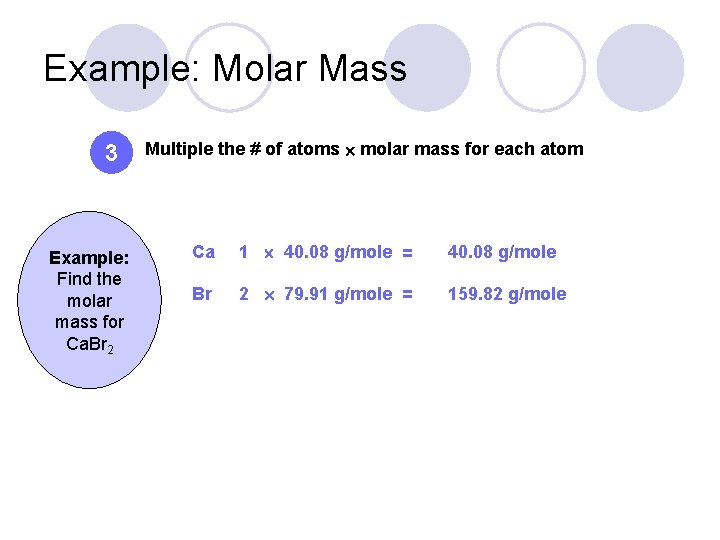
Example: Molar Mass 3 Example: Find the molar mass for Ca. Br 2 Multiple the # of atoms molar mass for each atom Ca 1 40. 08 g/mole = 40. 08 g/mole Br 2 79. 91 g/mole = 159. 82 g/mole
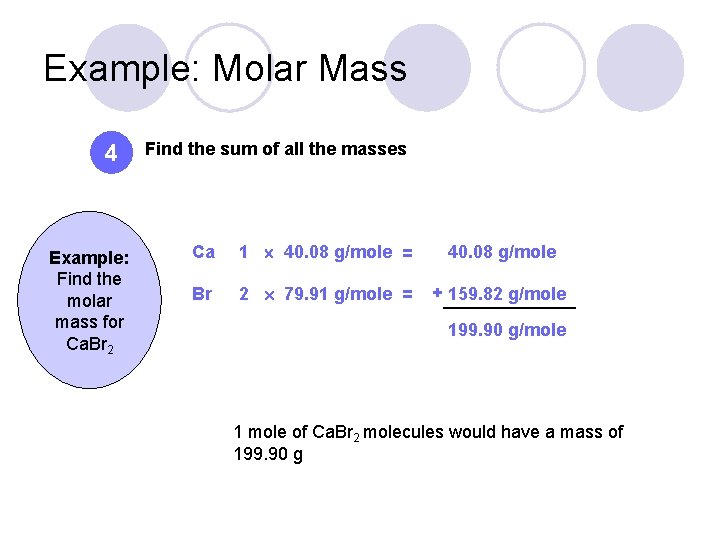
Example: Molar Mass 4 Example: Find the molar mass for Ca. Br 2 Find the sum of all the masses Ca 1 40. 08 g/mole = 40. 08 g/mole Br 2 79. 91 g/mole = + 159. 82 g/mole 199. 90 g/mole 1 mole of Ca. Br 2 molecules would have a mass of 199. 90 g
Example: Molar Mass & Parenthesis Be sure to distribute the subscript outside the parenthesis to each element inside the parenthesis. Example: Find the molar mass for Sr(NO 3)2

Example: Molar Mass & Parenthesis Be sure to distribute the subscript outside the parenthesis to each element inside the parenthesis. Example: Find the molar mass for Sr(NO 3)2 Sr 1 87. 62 g/mole = 87. 62 g/mole N 2 14. 01 g/mole = 28. 02 g/mole O 6 16. 00 g/mole = + 96. 00 g/mole 211. 64 g/mole 1 mole of Sr(NO 3)2 molecules would have a mass of 211. 64 g
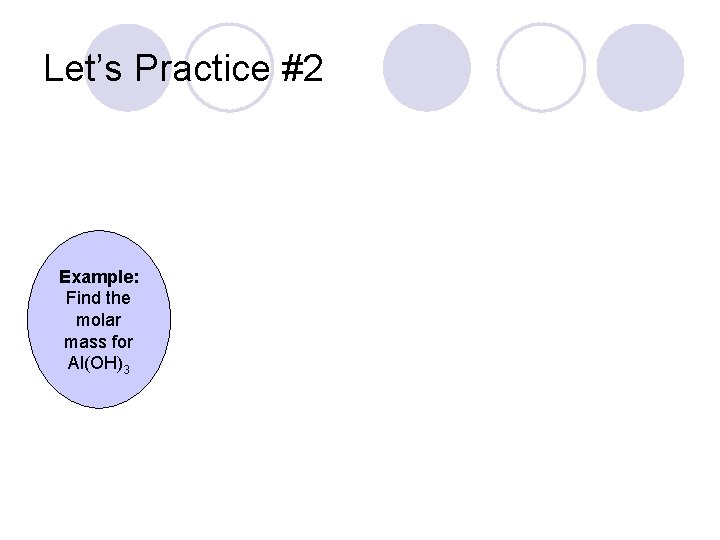
Let’s Practice #2 Example: Find the molar mass for Al(OH)3
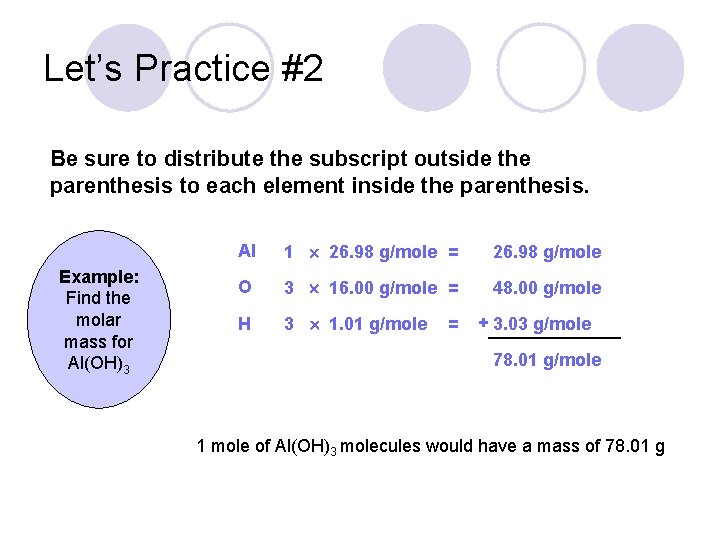
Let’s Practice #2 Be sure to distribute the subscript outside the parenthesis to each element inside the parenthesis. Example: Find the molar mass for Al(OH)3 Al 1 26. 98 g/mole = 26. 98 g/mole O 3 16. 00 g/mole = 48. 00 g/mole H 3 1. 01 g/mole = + 3. 03 g/mole 78. 01 g/mole 1 mole of Al(OH)3 molecules would have a mass of 78. 01 g
Using Molar Mass in Conversions

Example: Moles to Grams Example: How many grams are in 1. 25 moles of water?
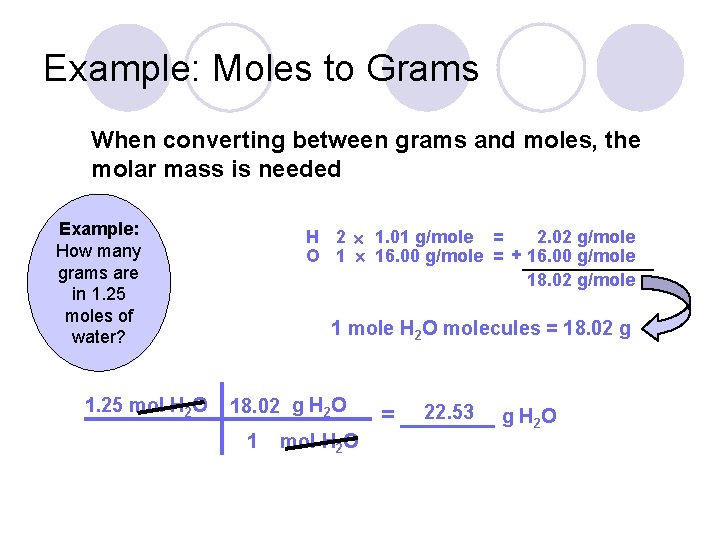
Example: Moles to Grams When converting between grams and moles, the molar mass is needed Example: How many grams are in 1. 25 moles of water? 1. 25 mol H 2 O H 2 1. 01 g/mole = 2. 02 g/mole O 1 16. 00 g/mole = + 16. 00 g/mole 18. 02 g/mole 1 mole H 2 O molecules = 18. 02 g H 2 O 1 mol H 2 O 22. 53 g H 2 O = _______
Let’s Practice #3 Example: How many moles are in 25. 5 g Na. Cl?
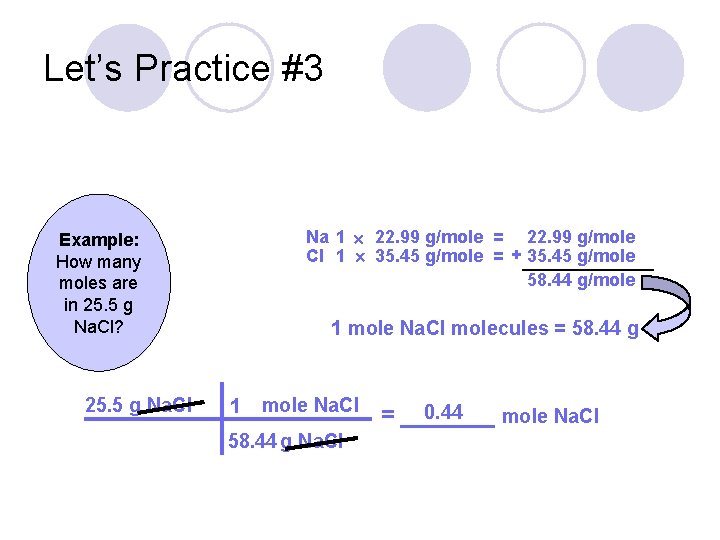
Let’s Practice #3 Na 1 22. 99 g/mole = 22. 99 g/mole Cl 1 35. 45 g/mole = + 35. 45 g/mole 58. 44 g/mole Example: How many moles are in 25. 5 g Na. Cl? 25. 5 g Na. Cl 1 mole Na. Cl molecules = 58. 44 g 1 mole Na. Cl 58. 44 g Na. Cl 0. 44 = _______ mole Na. Cl
Let’s Practice #4 Example: How many grams is a sample of 2. 75 × 1024 molecules of Sr. Cl 2?
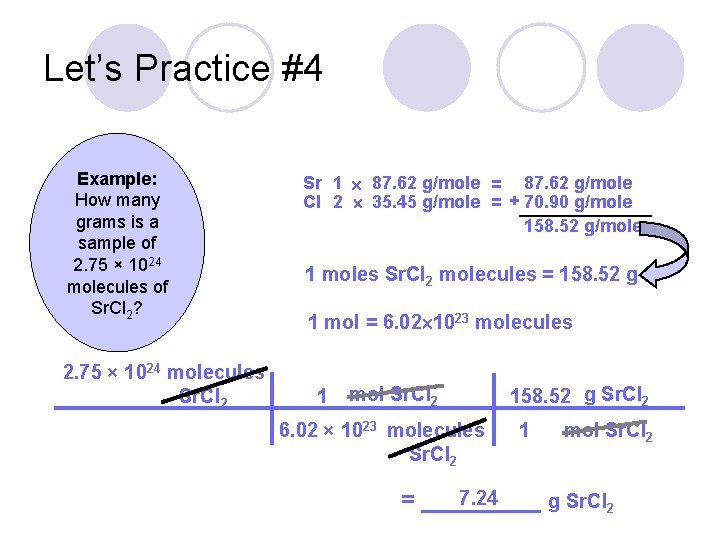
Let’s Practice #4 Example: How many grams is a sample of 2. 75 × 1024 molecules of Sr. Cl 2? 2. 75 × 1024 molecules Sr. Cl 2 Sr 1 87. 62 g/mole = 87. 62 g/mole Cl 2 35. 45 g/mole = + 70. 90 g/mole 158. 52 g/mole 1 moles Sr. Cl 2 molecules = 158. 52 g 1 mol = 6. 02 1023 molecules 1 mol Sr. Cl 2 6. 02 × 1023 molecules Sr. Cl 2 158. 52 g Sr. Cl 2 1 mol Sr. Cl 2 7. 24 = _____ g Sr. Cl 2
Example: Grams to Molecules Example: How many molecules are in 25. 5 g Na. Cl?
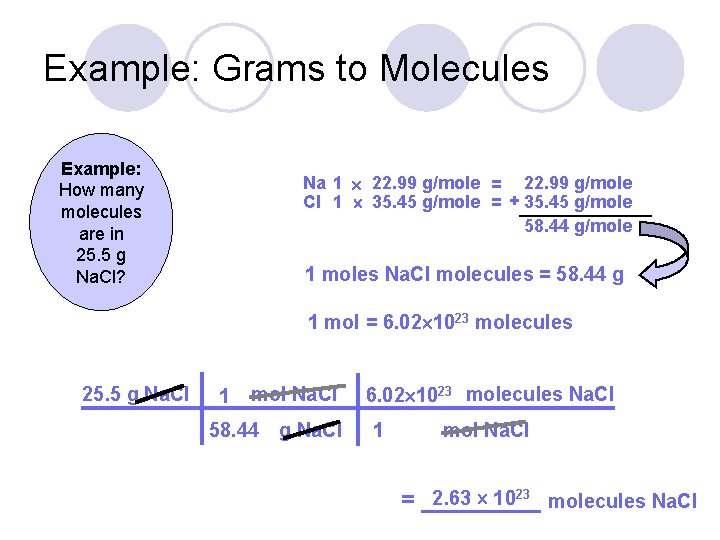
Example: Grams to Molecules Example: How many molecules are in 25. 5 g Na. Cl? Na 1 22. 99 g/mole = 22. 99 g/mole Cl 1 35. 45 g/mole = + 35. 45 g/mole 58. 44 g/mole 1 moles Na. Cl molecules = 58. 44 g 1 mol = 6. 02 1023 molecules 25. 5 g Na. Cl 1 mol Na. Cl 58. 44 g Na. Cl 6. 02 1023 molecules Na. Cl 1 mol Na. Cl 2. 63 1023 molecules Na. Cl = _____
- Slides: 33