4 2 The Periodic Table The periodic table
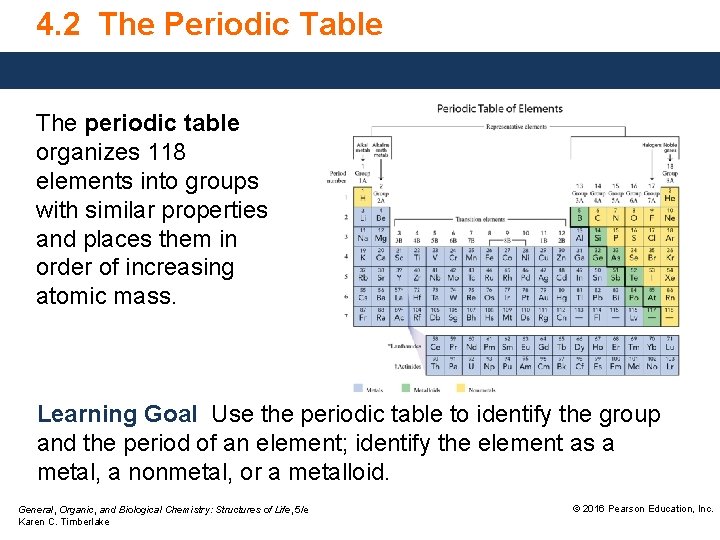
4. 2 The Periodic Table The periodic table organizes 118 elements into groups with similar properties and places them in order of increasing atomic mass. Learning Goal Use the periodic table to identify the group and the period of an element; identify the element as a metal, a nonmetal, or a metalloid. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
Groups and Periods In the periodic table, • elements are arranged according to properties. • groups contain elements with similar properties in vertical columns. • periods are horizontal rows of elements, counted from top to bottom of the table as Periods 1− 7. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
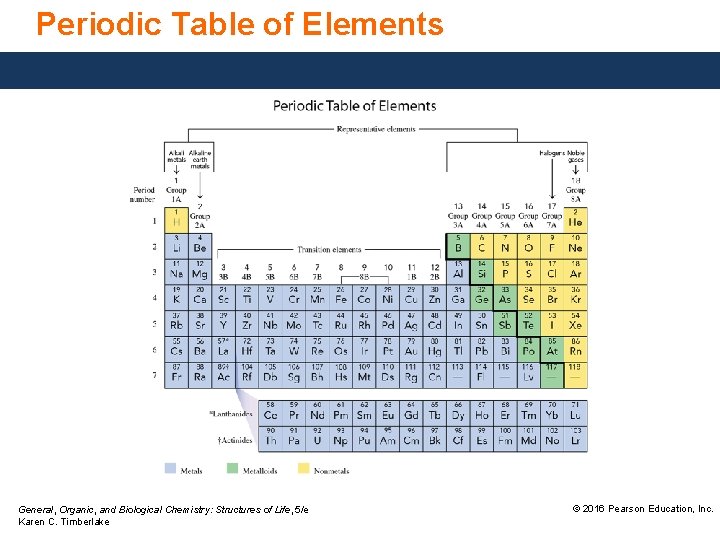
Periodic Table of Elements General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
Group Numbers Group numbers are written at the top of each vertical column. • Use the letter A for representative elements (Groups 1 A– 8 A). • Use the letter B for transition elements (Groups 3 B– 12 B). An alternative system uses numbers of 1– 18 for all of the groups, from left to right, across the periodic table. Because both systems are currently in use, they are both shown on the periodic table in this text and are included in our discussions of elements and group numbers. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
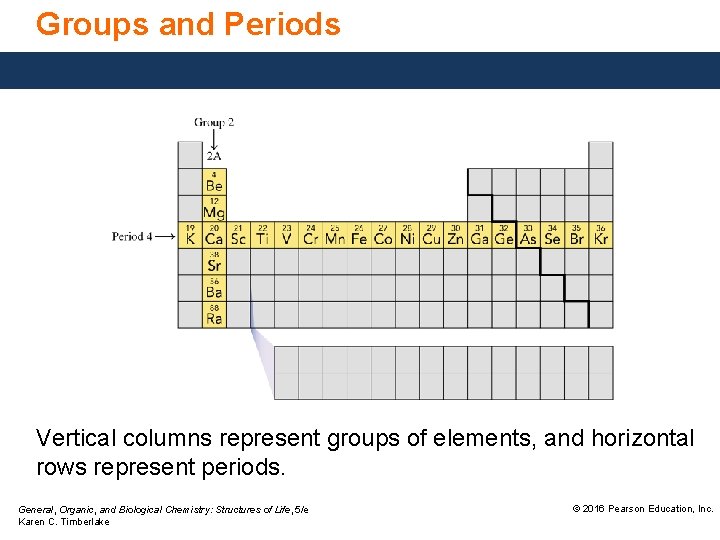
Groups and Periods Vertical columns represent groups of elements, and horizontal rows represent periods. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
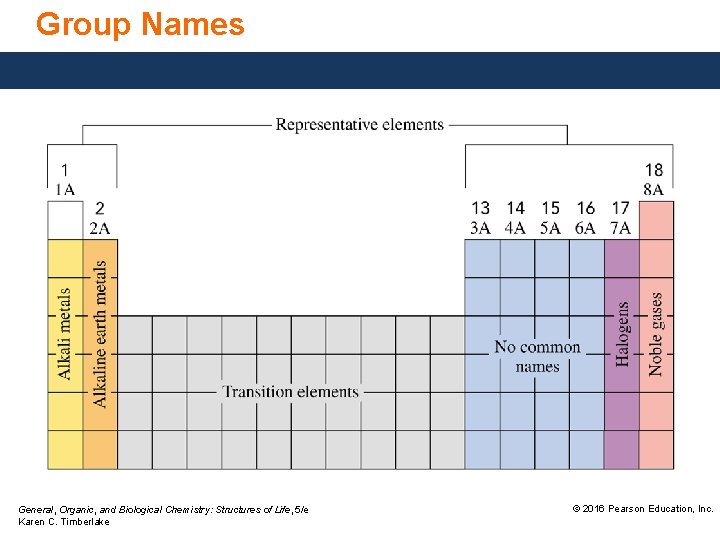
Group Names General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
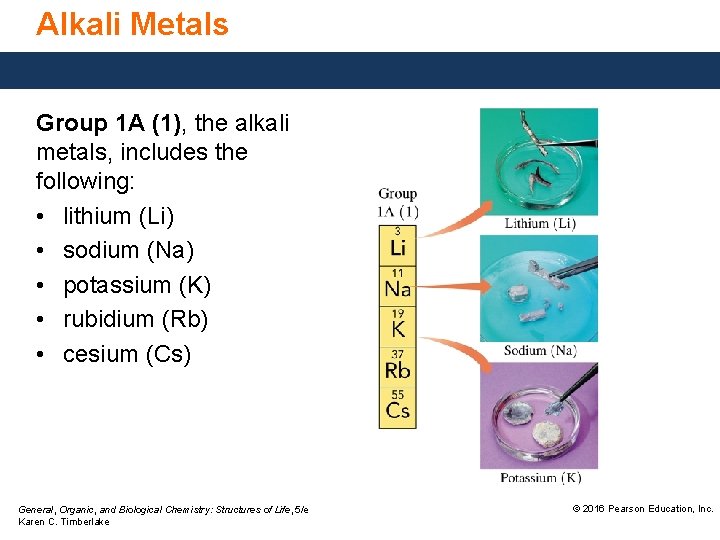
Alkali Metals Group 1 A (1), the alkali metals, includes the following: • lithium (Li) • sodium (Na) • potassium (K) • rubidium (Rb) • cesium (Cs) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Alkaline Earth Metals Group 2 A (2) elements, the alkaline earth metals, are shiny but not as reactive as Group 1 A metals. They include the following: • beryllium (Be) • magnesium (Mg) • calcium (Ca) • strontium (Sr) • barium (Ba) • radium (Ra) Strontium gives the red color in fireworks. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
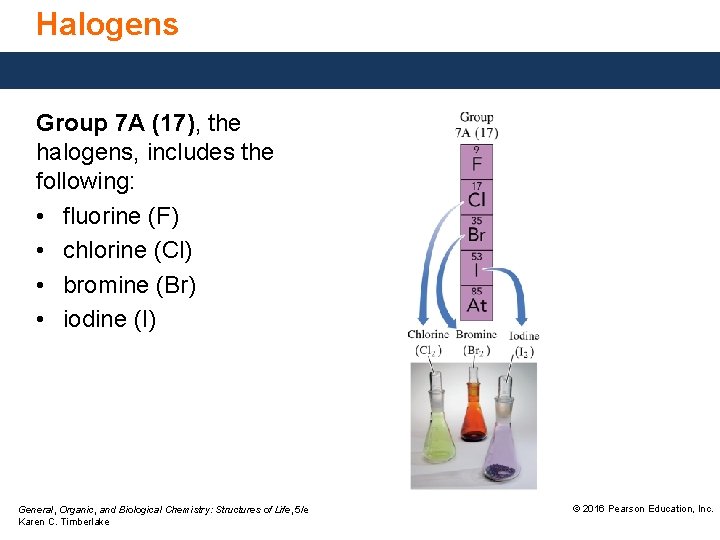
Halogens Group 7 A (17), the halogens, includes the following: • fluorine (F) • chlorine (Cl) • bromine (Br) • iodine (I) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
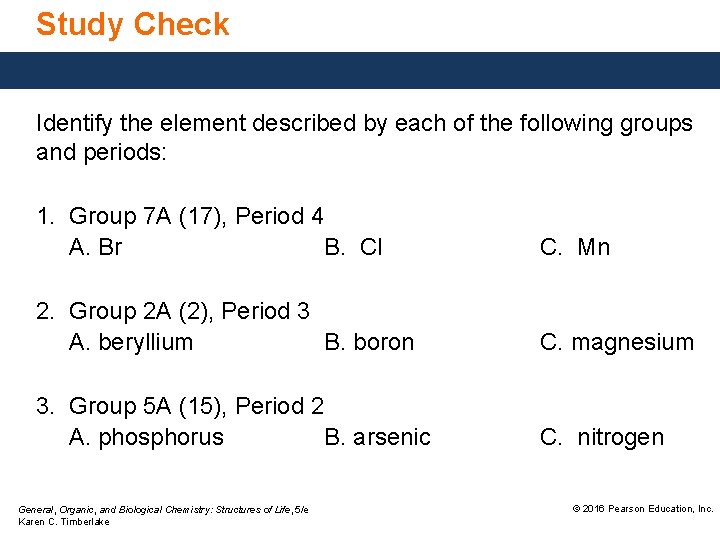
Study Check Identify the element described by each of the following groups and periods: 1. Group 7 A (17), Period 4 A. Br B. Cl C. Mn 2. Group 2 A (2), Period 3 A. beryllium B. boron C. magnesium 3. Group 5 A (15), Period 2 A. phosphorus B. arsenic C. nitrogen General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
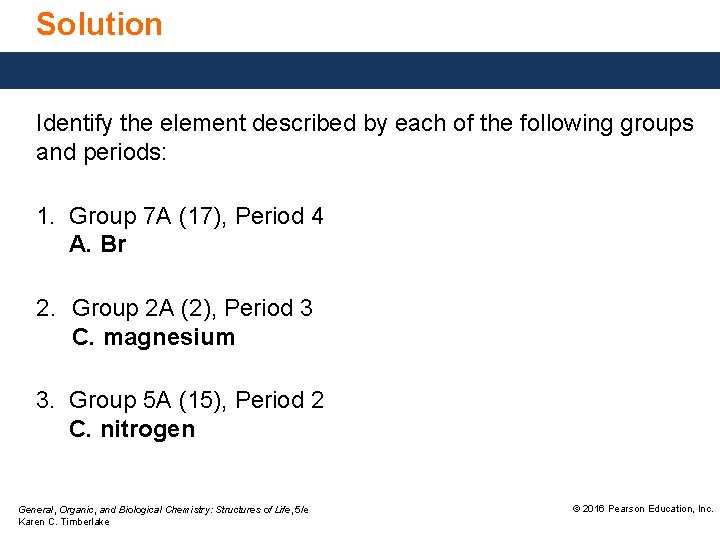
Solution Identify the element described by each of the following groups and periods: 1. Group 7 A (17), Period 4 A. Br 2. Group 2 A (2), Period 3 C. magnesium 3. Group 5 A (15), Period 2 C. nitrogen General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
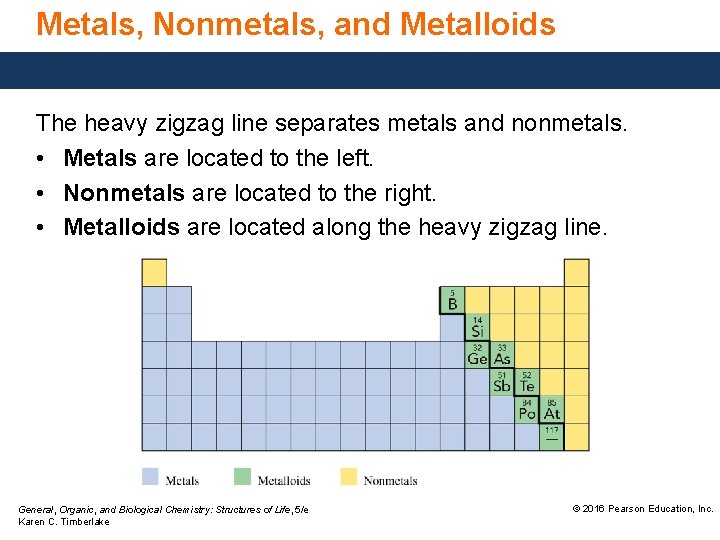
Metals, Nonmetals, and Metalloids The heavy zigzag line separates metals and nonmetals. • Metals are located to the left. • Nonmetals are located to the right. • Metalloids are located along the heavy zigzag line. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Characteristics of Metals, Nonmetals, and Metalloids Metals, except for hydrogen, located on the left of the periodic table, • are shiny and ductile, and conduct heat and electricity. • are solids, except for mercury (Hg), which is a liquid. Nonmetals, located on the right side of the periodic table, • are dull, brittle, and poor conductors but often good insulators. • have low densities and melting points. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
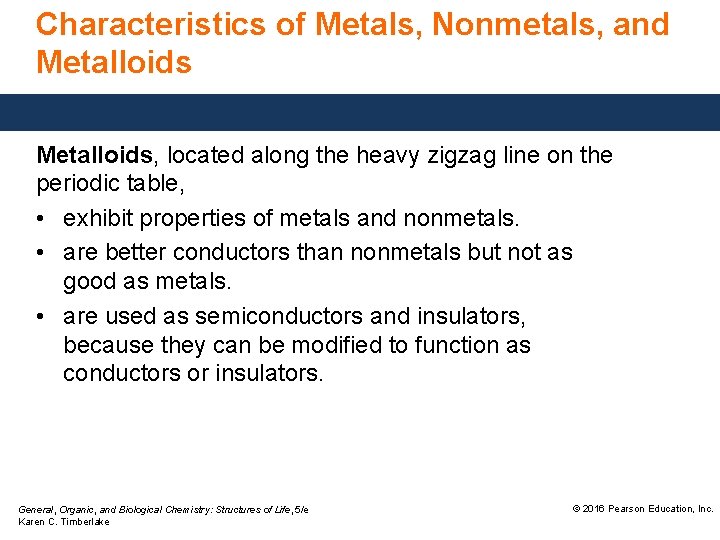
Characteristics of Metals, Nonmetals, and Metalloids, located along the heavy zigzag line on the periodic table, • exhibit properties of metals and nonmetals. • are better conductors than nonmetals but not as good as metals. • are used as semiconductors and insulators, because they can be modified to function as conductors or insulators. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
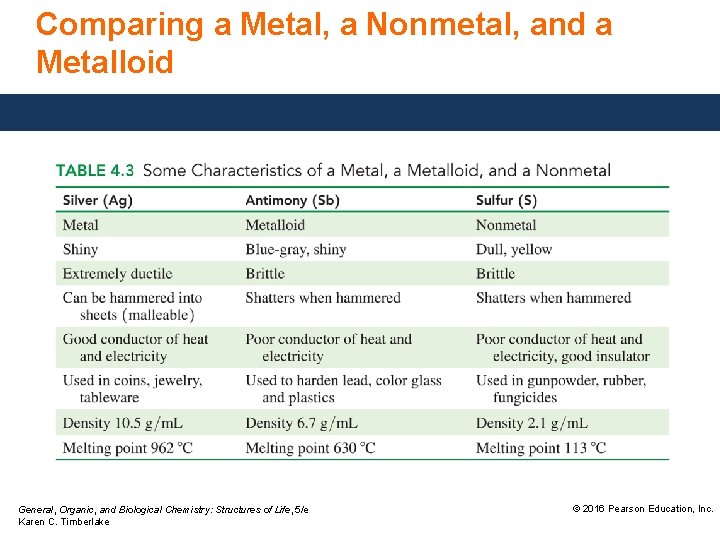
Comparing a Metal, a Nonmetal, and a Metalloid General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
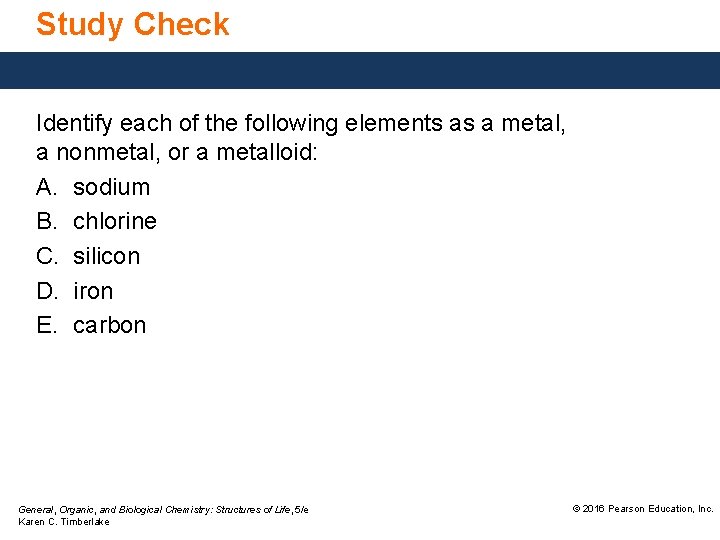
Study Check Identify each of the following elements as a metal, a nonmetal, or a metalloid: A. sodium B. chlorine C. silicon D. iron E. carbon General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
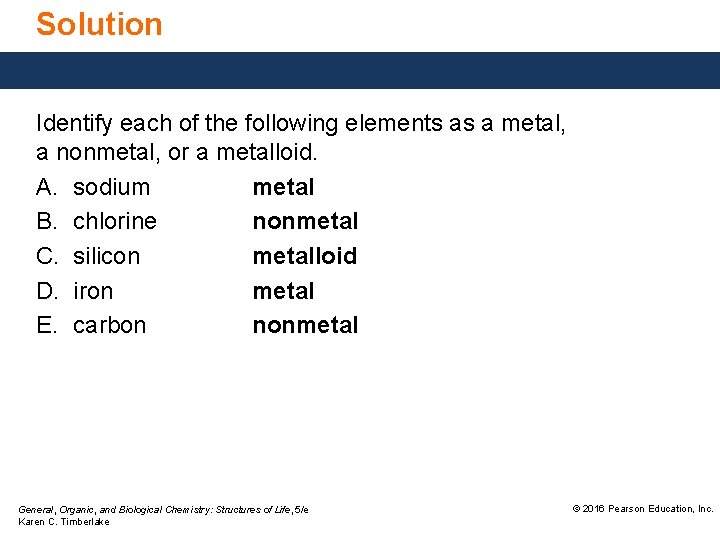
Solution Identify each of the following elements as a metal, a nonmetal, or a metalloid. A. sodium metal B. chlorine nonmetal C. silicon metalloid D. iron metal E. carbon nonmetal General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
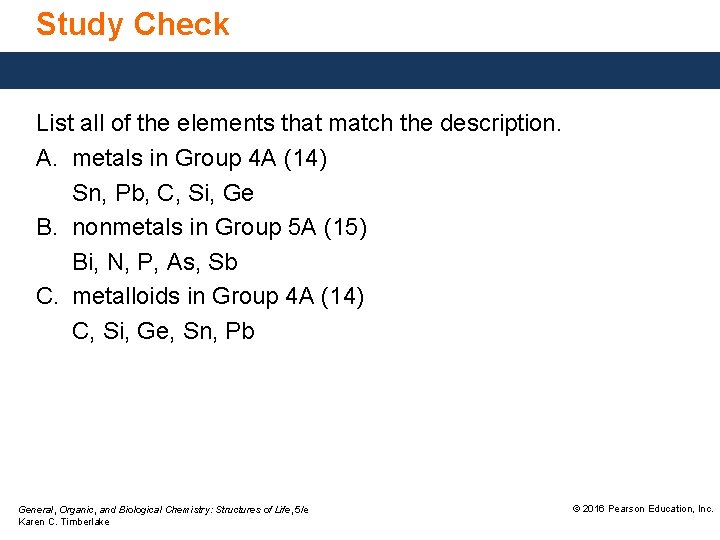
Study Check List all of the elements that match the description. A. metals in Group 4 A (14) Sn, Pb, C, Si, Ge B. nonmetals in Group 5 A (15) Bi, N, P, As, Sb C. metalloids in Group 4 A (14) C, Si, Ge, Sn, Pb General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
Solution List all of the elements that match the description. A. metals in Group 4 A (14) Sn, Pb B. nonmetals in Group 5 A (15) N, P C. metalloids in Group 4 A (14) Si, Ge General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
Chemistry Link to Health: Elements Essential to Health Of all the elements, • 20 are essential for the well-being and survival of the human body. • four—oxygen, carbon, hydrogen, and nitrogen—make up 96% of our body mass. • most of our hydrogen and oxygen is found as water, which makes up 55 to 60% of our body mass. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
Chemistry Link to Health: Elements Essential to Health Macrominerals—Ca, P, K, Cl, S, Na, and Mg—are representative elements involved in • the formation of bones and teeth. • maintenance of heart and blood vessels, muscle contraction, nerve impulses, and acid–base balance of body fluids. • regulation of cellular metabolism. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
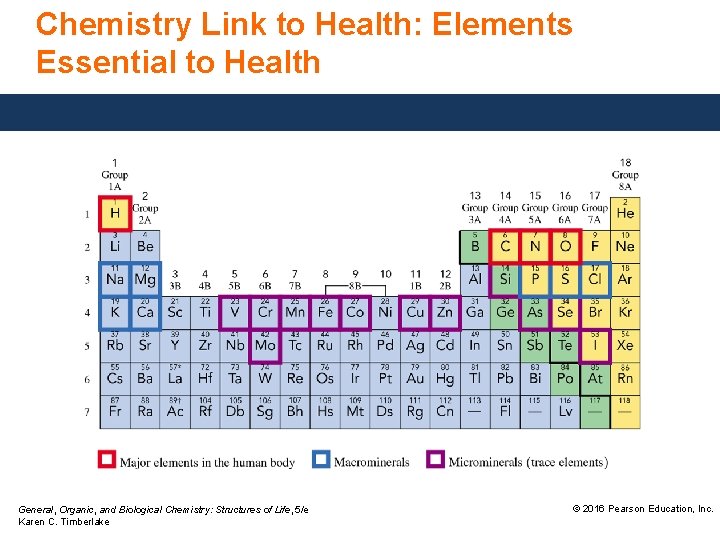
Chemistry Link to Health: Elements Essential to Health General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.
- Slides: 22