4 2 The Periodic Table Dmitri Mendeleev Russian

4. 2 The Periodic Table

Dmitri Mendeleev • Russian scientist that first arranged all the known elements into rows and columns with similar properties in 1869.
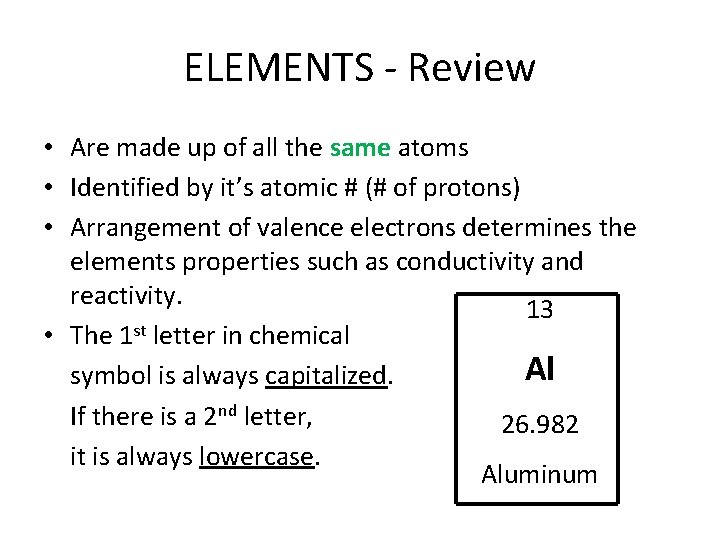
ELEMENTS - Review • Are made up of all the same atoms • Identified by it’s atomic # (# of protons) • Arrangement of valence electrons determines the elements properties such as conductivity and reactivity. 13 • The 1 st letter in chemical Al symbol is always capitalized. If there is a 2 nd letter, 26. 982 it is always lowercase. Aluminum
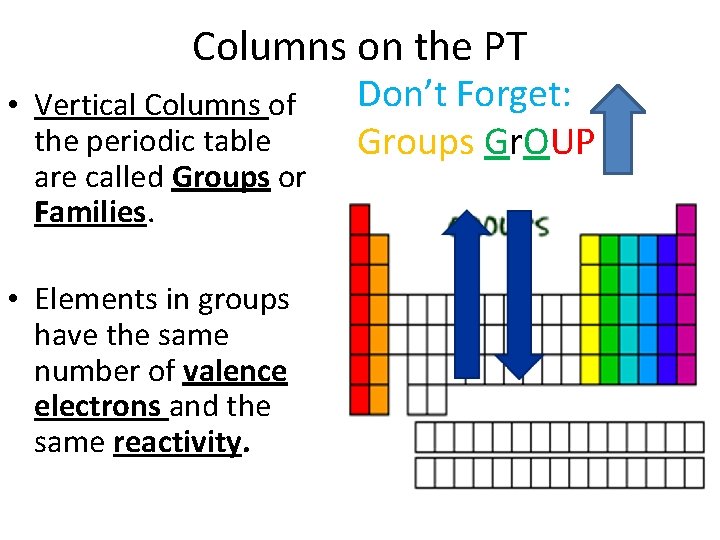
Columns on the PT • Vertical Columns of the periodic table are called Groups or Families. • Elements in groups have the same number of valence electrons and the same reactivity. Don’t Forget: Groups Gr. OUP
Rows on the PT • Horizontal rows on the periodic table are called Periods. • Rows of the periodic table have elements with the same number of energy levels.
Groups vs. Periods • Go up and down like • Left to right (rows) a family tree • Have the same number of energy number of valence rings/levels in each electrons row • Have same chemical properties (reactivity)
Parts of the PT & characteristics • The Periodic Table is organized by valence electrons, energy levels, atomic number, and atomic mass. • Because of this organization, there are other patterns we can now identify. • The PT can also be organized into 3 main categories: – Metals – Nonmetals – Metalloids • Elements in each of these category share the SAME physical properties.

What is a Physical Property? • A characteristic that can be observed or measured without changing the identity (composition) of the substance.
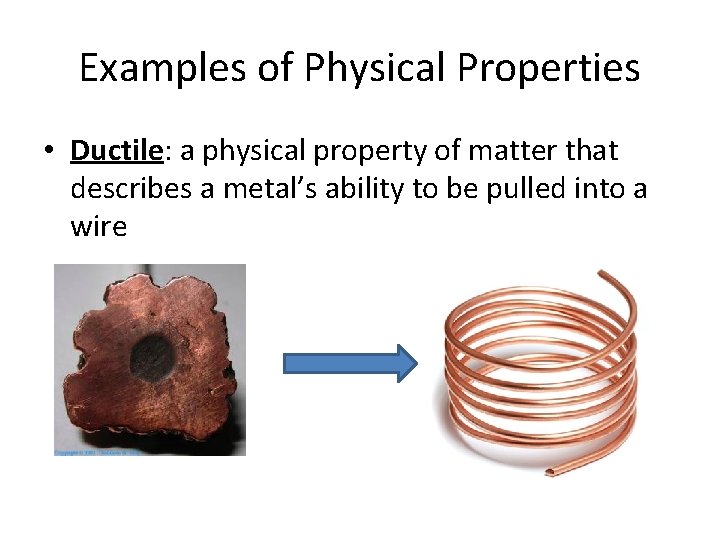
Examples of Physical Properties • Ductile: a physical property of matter that describes a metal’s ability to be pulled into a wire

Examples of Physical Properties • **Malleability**: a physical property of matter that describes the ability to change shape without breaking or being hammered into thin sheets The opposite of malleable is brittle (breaks easily. )
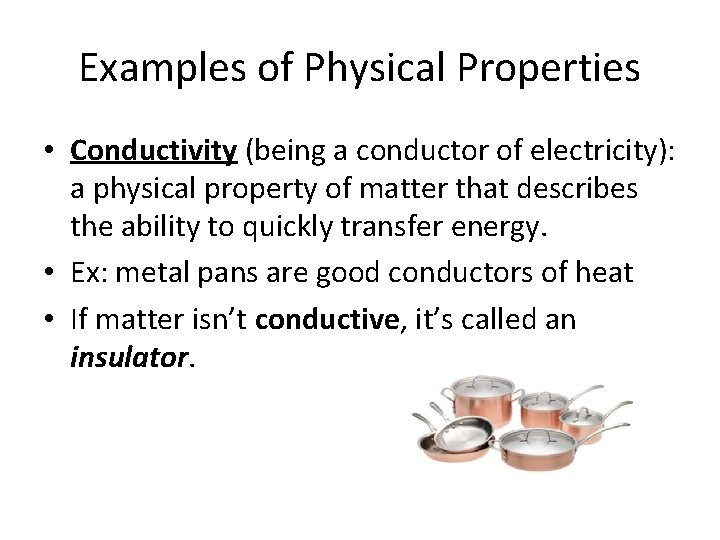
Examples of Physical Properties • Conductivity (being a conductor of electricity): a physical property of matter that describes the ability to quickly transfer energy. • Ex: metal pans are good conductors of heat • If matter isn’t conductive, it’s called an insulator.

Examples of Physical Properties • Luster: a physical property of matter that describes the way light is reflected • Ex: shiny rocks or metal • Opposite of shiny is dull. (Like this rock. )

Metals • Metals are located to the LEFT of the zigzag line. • Metals are: – Malleable: hammered into sheets – Ductile: can be stretched into wire – Have Luster: shiny – conductors of heat and electricity – magnetic – Solid at room temperature • The 1 exception is hydrogen.
Nonmetals • Nonmetals are located to the RIGHT of the zigzag line. (Exception: Hydrogen) • Nonmetals are: – Brittle: break easily – Dull: not shiny – Insulators: poor conductors of heat and electricity
Metalloids • Metalloids are located along the borders of the zigzag line. (Exception: Aluminum) • **Metalloids have properties similar to BOTH metals and nonmetals. – Semi-conductors (can be a conductor or insulator) – May be dull or shiny – May be brittle or malleable
- Slides: 15